Abstract
Inositol trisphosphate (IP3) is a Ca2+-mobilizing second messenger shown to modulate atrial muscle contraction and is thought to contribute to atrial fibrillation. Cellular pathways underlying IP3 actions in cardiac tissue remain poorly understood, and the work presented here addresses the question whether IP3-mediated Ca2+ release from the sarcoplasmic reticulum is linked to adenylyl cyclase activity including Ca2+-stimulated adenylyl cyclases (AC1 and AC8) that are selectively expressed in atria and sinoatrial node (SAN). Immunocytochemistry in guinea pig atrial myocytes identified colocalization of type 2 IP3 receptors with AC8, while AC1 was located in close vicinity. Intracellular photorelease of IP3 by UV light significantly enhanced the amplitude of the Ca2+ transient (CaT) evoked by electrical stimulation of atrial myocytes (31 ± 6% increase 60 s after photorelease, n = 16). The increase in CaT amplitude was abolished by inhibitors of adenylyl cyclases (MDL-12,330) or protein kinase A (H89), showing that cAMP signaling is required for this effect of photoreleased IP3. In mouse, spontaneously beating right atrial preparations, phenylephrine, an α-adrenoceptor agonist with effects that depend on IP3-mediated Ca2+ release, increased the maximum beating rate by 14.7 ± 0.5%, n = 10. This effect was substantially reduced by 2.5 µmol/L 2-aminoethyl diphenylborinate and abolished by a low dose of MDL-12,330, observations which are again consistent with a functional interaction between IP3 and cAMP signaling involving Ca2+ stimulation of adenylyl cyclases in the SAN pacemaker. Understanding the interaction between IP3 receptor pathways and Ca2+-stimulated adenylyl cyclases provides important insights concerning acute mechanisms for initiation of atrial arrhythmias.
NEW & NOTEWORTHY This study provides evidence supporting the proposal that IP3 signaling in cardiac atria and sinoatrial node involves stimulation of Ca2+-activated adenylyl cyclases (AC1 and AC8) by IP3-evoked Ca2+ release from junctional sarcoplasmic reticulum. AC8 and IP3 receptors are shown to be located close together, while AC1 is nearby. Greater understanding of these novel aspects of the IP3 signal transduction mechanism is important for future study in atrial physiology and pathophysiology, particularly atrial fibrillation.
Keywords: adenylyl cyclase, cardiac atria, Ca2+ signaling, cyclic AMP, inositol trisphosphate
INTRODUCTION
Ca2+ handling in the heart is vital to normal physiological function and regulation of excitation-contraction coupling (1, 2). Ca2+ signaling in cardiomyocytes is tightly regulated, for example, by protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase II (CaMKII), or by Ca2+-mobilizing elements such as inositol trisphosphate (IP3), cADP-ribose (cADPR), and nicotinic acid adenine dinucleotide phosphate (NAADP), as well as by Ca2+ itself (1–3). The atrial and ventricular chambers of the heart have very different functions, and therefore it is not surprising that there are many differences between atrial and ventricular myocytes in excitation-contraction coupling and in the handling of Ca2+ ions by different intracellular compartments. One characteristic feature of atrial myocytes is the relative abundance of receptors for inositol trisphosphate (IP3) compared with ventricular myocytes (3). IP3 is a Ca2+-mobilizing second messenger (4) that acts to open IP3 receptors (IP3R), located on the sarcoplasmic reticulum (SR) of cardiomyocytes (3, 5). IP3 is positively inotropic in atrial (3) and ventricular (6) preparations and is positively chronotropic in the sinoatrial node (SAN) (7, 8). IP3 is synthesized upon stimulation of phospholipase C (PLC) commonly, but not exclusively, by G-protein coupled receptors associated with Gq (9). In cardiac myocytes, endothelin-1 (ET-1), angiotensin II (Ang-II), and phenylephrine (PE) all increase intracellular IP3 level (10) via their actions at the Gq-coupled ET-A, Ang-II, and α-adrenergic receptors, respectively.
Early functional studies revealed a much greater effect of IP3-associated stimuli on the contractility of atrial preparations than upon their ventricular counterparts (11), and expression of IP3R type 2 (IP3R2) is now known to be at least six times greater in atrial myocytes (3). IP3R expression is significantly increased during atrial fibrillation in both human patients (12) and animal models (13). Inhibiting Gq-coupled Ang-II receptors has been shown to prevent the early remodeling associated with rapid atrial pacing (14), whereas in healthy atrial myocytes, Gq-associated signaling causes an IP3R-dependent increase in the Ca2+ spark rate of quiescent myocytes and amplitude of the stimulated Ca2+ transient (15), effects matched on direct application of IP3 [Ca2+ sparks (3, 16), Ca2+ transients (3)]. Interestingly, even in healthy cells, IP3-dependent stimulation can be associated with the generation of spontaneous diastolic Ca2+ events (3, 5).
Cardiac function is also controlled by pathways involving production of cAMP (1, 17). These pathways are commonly thought to be distinct and separate from the previously mentioned IP3-dependent mechanisms, although evidence supports a cAMP-mediated regulation of the sensitivity of IP3Rs to Ca2+ (18, 19). Here, we consider a possible novel link between cAMP-dependent mechanisms and IP3-mediated Ca2+ release from the SR, which arises from the existence and location of the Ca2+-stimulated isoforms of adenylyl cyclase, AC1 and AC8, which have been shown to be selectively expressed in SAN (20, 21) and atria (22), whereas in contrast, ventricular myocytes predominantly express AC5 and AC6 (23), which lack Ca2+ sensitivity. Interestingly, AC1 and AC8, like IP3R, have been shown to be expressed close to the surface of SA node and atrial myocytes, although the relative positions of these adenylyl cyclases and IP3Rs have not been previously explored (20).
Functionally, Ca2+-stimulated adenylyl cyclases have been shown to play a role in regulating pacemaker activity (8, 24), at least in part by controlling the synthesis of cAMP, which can directly regulate an important pacemaker current, I(f) (25), independently of basal PKA activity (26) and Ca2+/CAMKII-dependent phosphorylation (27). Expression of Ca2+-stimulated AC1 has also been shown to enhance beating rate in hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2)-mediated “biological pacemakers” (28).
Previous experiments also provided evidence for a role for Ca2+-stimulated adenylyl cyclases in regulating Ca2+ transients and L-type Ca2+ currents in atrial myocytes (22). In particular, loading atrial myocytes with the Ca2+ chelator, BAPTA, was shown to cause a substantial reduction of the amplitude of L-type Ca2+ currents in atrial myocytes, but BAPTA was without effect when cytosolic cAMP levels were maintained at 200 μM by application from a patch pipette. These observations were interpreted as evidence for ongoing enhancement of L-type Ca2+ currents, which was dependent on basal activity of Ca2+-stimulated adenylyl cyclases in atrial myocytes. This interpretation was further supported by the observations that the amplitudes of L-type Ca2+ currents were also reduced when adenylyl cyclases were inhibited by MDL12-330A [cis-N-(2-phenylcyclopentyl)-azacyclotridec-1-en-2-amine hydrochloride] (22).
In view of the functional importance of Ca2+-stimulated adenylyl cyclases in regulating atrial and SAN function, and the likelihood that IP3Rs may be located close to AC1 and AC8, the hypothesis to be tested is that actions of IP3 in atria and SAN depend on IP3-mediated Ca2+ release from the SR leading to activation of the adenylyl cyclases, AC1 and AC8.
METHODS
All animal experiments were performed in accordance with the United Kingdom Home Office Guide on the Operation of Animal (Scientific Procedures) Act of 1986. All experimental protocols (Schedule 1) were approved by the University of Oxford, Procedures Establishment License (PEL) Number XEC303F12.
Atrial Myocyte Isolation
Male Dunkin Hartley guinea pigs (350–550 g, Envigo, UK) were housed and maintained in a 12-h light/dark cycle with ad libitum access to standard diet and sterilized water. Guinea pigs were culled by cervical dislocation in accordance with Home Office Guidance on the Animals (Scientific Procedures) Act (1986). Atrial myocytes were isolated following the method of Collins et al. (2011) (29) and stored at 4°C in a high potassium medium containing (in mmol/L): KCl 70, MgCl2 5, K+ glutamine 5, taurine 20, EGTA 0.1, succinic acid 5, KH2PO4 20, HEPES 5, glucose 10 at pH 7.2 with KOH. Healthy atrial myocytes were identified on the basis of morphology.
Immunocytochemistry
Immunocytochemistry staining and analysis was carried out using the method of Collins and Terrar (2012) (22). AC1 (sc25743) and AC8 (sc32128) primary antibodies were purchased commercially (Santa Cruz Biotechnology, Santa Cruz, CA) and used at a dilution of 1:200. Specificity of sc25743 and sc32128 for AC1 and AC8 was confirmed by Western blot [methods and data previously published in Mattick et al. (20)]. IP3R monoclonal primary antibodies (IP3R1 KM1112, IP3R2 KM1083, IP3R3 KM1082) were a kind gift from Professor Katsuhiko Mikoshiba (30) and used at a dilution of 1:1,000. The specificity of antibodies KM1112, KM1083, and KM1082 has been previously verified using Western blot by Sugiyama et al. (31) as well as in previous publications (30). Use of these IP3R antibodies has been extensively covered in previous studies (32–35). All primary antibody staining was carried out overnight at 4°C. Secondary antibody labeling was carried out using either Alexa Fluor 488 or 555 conjugated secondary antibodies (Invitrogen, UK), raised against the appropriate species, for 60 min at room temperature at a dilution of 1:400. Observations were carried out using a Zeiss LSM 510 confocal microscope (×40 or ×63 oil objectives). For detection of Alexa Fluor 488, fluorescence excitation was at 488 nm with emission collected at 505–530 nm. An excitation filter of 543 nm and an emission filter at >560 nm were used to detect Alexa Fluor 555. The two channels were imaged sequentially. Control cells where the primary or secondary antibody was to be excluded were incubated with 5% donkey serum alone without addition of the relevant antibody. To quantify the relationship between the red and green signals that were imaged during double labeling experiments, we carried out a pixel-by-pixel colocalization analysis on whole cells in ImageJ [using the plugin “Just Another Co-localization Plugin” (36)]. The analysis assessed, pixel by pixel across the whole image, the correlation between intensity values of the two dyes viewed as grayscale images and used produced Pearson’s coefficient, which is between −1 (total exclusion of the signals) and +1 (complete colocalization of the signals).
Ca2+ Transient Imaging and IP3 Photorelease
For whole cell fluorescence experiments, isolated atrial myocytes were incubated with Fluo-5F (3 µmol/L) for 10 min, then plated to a glass coverslip for imaging. Carbon fiber electrodes were used to field-stimulate Ca2+ transients at a rate of 1 Hz. All experiments were carried out at 35 ± 2°C (fluctuation within a single experiment was <0.5°C) under gravity-fed superfusion of physiological salt solution (PSS, in mmol/L): NaCl 125, NaHCO3 25, KCl 5.4, NaH2PO4 1.2, MgCl2 1, glucose 5.5, CaCl2 1.8, oxygenated with 95% O2-5% CO2 (solution pH 7.4 after oxygenation and heating). Solution flow rate was 3 mL/min.
For photorelease experiments, isolated atrial myocytes were incubated for 60 min at room temperature with 0.5 µmol/L membrane-permeant caged IP3 (caged-IP3/PM) and 0.025% pluronic F127 (Enzo Life Sciences, UK). Fluo-5F-AM (3 µmol/L) was added for the last 10 min of incubation. DMSO concentrations were 0.5% during IP3/PM loading and 0.75% during IP3/PM + Fluo-5F. Cells were visualized using a Zeiss Axiovert 200 with attached Nipkow spinning disk confocal unit (CSU-10, Yokogawa Electric Corporation, Japan). Excitation light, transmitted through the CSU-10, was provided by a 488-nm diode laser (Vortran Laser Technology Inc., Sacramento, CA). Emitted light was passed through the CSU-10 and collected by an iXON897 EM-CCD camera (Oxford Instruments, UK) at 60 frames per second with 2 × 2 binning (pixel size = 0.66667 µm2). UV uncaging was carried out using 3× rapid flashes of a Xenon arc lamp (Rapp Optoelectronics, Germany), delivered through the objective lens. To avoid dye bleaching, the cells were not continually exposed to 488 nm light. Instead, a video of 8–10 s of calcium transients was recorded at a number of timepoints immediately before photorelease (denoted 0 s) and at the indicated times after UV exposure. Calcium transients were measured using regions of interests (ROIs) in Andor iQ software (v 1.7) to record average whole cell fluorescence. For inhibitor work, each aliquot of IP3/PM (3–4 experiments) was first used for a control experiment and inhibitor data were excluded if control cells did not respond. Cells were also excluded if, upon analysis, control (pre-photorelease) data exhibited alternans, missed beats, or were otherwise unstable. Adenylyl cyclases were inhibited using the nonselective adenylyl cyclase inhibitor MDL 12–330 A (3 µmol/L) (37), whereas for inhibition of PKA, we used H89 (1 µmol/L). 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10 µmol/L) and Nω-nitro-L-arginine methyl ester(L-NAME; 100 µmol/L) were used to inhibit soluble guanylyl cyclase and nitric oxide synthase, respectively. All inhibitors were sourced from Tocris Bioscience (UK), except L-NAME which was sourced from Sigma-Aldrich, and applied for at least 10 min, of which at least 5 min were stimulated at 1 Hz. Calcium transient time courses were analyzed in ClampFit (v 10.4) and rise and decay curve-fitting were carried out by fitting single exponentials using Prism (v 8).
Murine Atrial Studies
Adult male CD1 mice (CD-1 IGS 30–35 g, Charles River, UK) were housed maintained in a 12-h light/dark cycle with ad libitum access to standard diet and sterilized water. Mice were culled by cervical dislocation in accordance with Home Office Guidance on the Animals (Scientific Procedures) Act (1986). The heart was rapidly excised and washed in heparin-containing PSS. The ventricles were dissected away under a microscope, and the area adjacent to the sinoatrial node was cleared of connective tissue. The spontaneously beating atrial preparation was mounted in a 37°C organ bath containing oxygenated PSS and connected to a force transducer (MLT0201 series, ADInstruments, UK) to visualize contractions. Resting tension was set between 0.2 and 0.3 g, the tension signal was low-pass filtered at 20 Hz, and beating rate was calculated from the time interval between contractions. After stabilization (variation in average rate of a 10-s sample of no more than 2 beats/min over a 10-min period), cumulative concentrations of PE were added to the bath (range 0.1–30 µmol/L) in the presence of metoprolol (1 µmol/L, applied 30 min before PE) to ensure specificity to α-adrenergic effects. Preparations were excluded if stabilized beating rate under control conditions (PSS only) was less than 300 beats/min or if preparations were not rhythmic. In addition to the inhibitors listed previously, IP3 receptors were inhibited using 2-aminoethyl diphenylborinate (2-APB) (2.5 µmol/L, Merck, UK) (37). AC1 was inhibited using the AC1 selective inhibitor ST034307 (1 µmol/L, Tocris, UK) (38), whereas U73122 (5 µmol/L, Tocris, UK) was used to inhibit IP3 production by PLC. Inhibitors were added after stabilization of the preparations and applied for either 30 min (2-APB, ODQ, L-NAME, U73122, and ST034307) or 60 min (MDL, H89) before PE additions. PE dose-response curves were started only after tissue had reached a stable response, where any occurred.
Statistics
For all single-cell data, t tests or ANOVA were used as appropriate with Dunnett’s or Tukey’s post hoc test to compare groups to a single control or to all other groups, respectively, as required. Experimenters were not blinded to the conditions being analyzed. Log(concentration)-response curves, used to estimate EC50s and maximum responses, were calculated using Prism v8.4.0 software (GraphPad, CA), by fitting an agonist-response curve with a fixed slope to normalized response data. Normalized data were used to compare responses as it was expected some inhibitors used would significantly affect the control beating rate or Ca2+ transient amplitude. Fitted values were compared using ANOVA with Dunnett’s or Tukey’s post hoc test. For analysis of Ca2+ transient rise and decay times at 0 s and 120 s after IP3 photorelease, Ca2+ data were analyzed using pClamp v10 (Molecular Devices, CA) to generate times corresponding to 10%–90% and 10%–50% rise time and 90%-10%, 90%-75%, 90%-50%, and half-width decay time. Decay phases of transients were also fitted using one phase decay least squares regression (Prism v8.4.0). Data are presented as means ± SE of recorded values, other than dose-response curve maximum, which is given as means ± SE of best-fit value, and EC50, which is presented as best-fit value with 95% confidence interval.
RESULTS
Type 2 IP3 Receptors are Colocalized with AC8 in Cardiac Atrial Myocytes
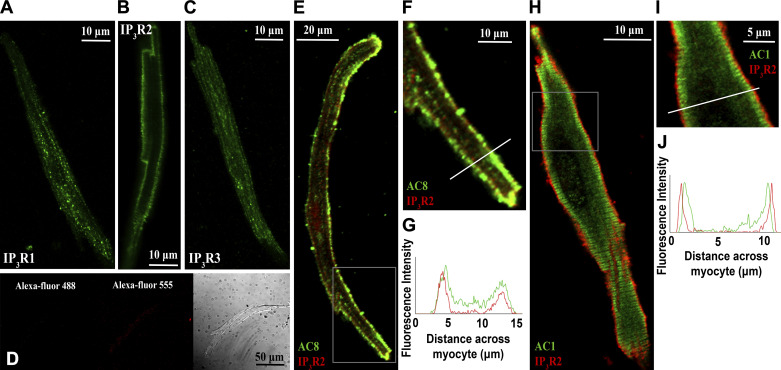
In agreement with published literature (3), type 2 IP3 receptors (IP3R2) were visualized in a punctate pattern at the cell periphery, consistent with a position on junctional SR (Fig. 1B). The vast majority of IP3R expression in cardiac atrial myocytes is thought to be IP3R2, with IP3R1 and IP3R3 as minimal components (3). Consistent with this notion, staining for IP3R1 (Fig. 1A) and IP3R3 (Fig. 1C) receptors did not demonstrate a distinct subcellular pattern and may represent at least some nonspecific labeling. Negative controls for immunocytochemistry (application of secondary antibody only) are shown in Fig. 1D.
Figure 1.
IP3R2 colocalizes with AC8 in guinea pig atrial myocytes. A–C: representative examples of fixed, isolated guinea pig atrial myocytes labeled for IP3R1 (A), IP3R2 (B), IP3R3 (C), and negative controls (D). E: representative example of a fixed, isolated guinea pig atrial myocyte co-immunolabeled for IP3R2 (red) and AC8 (green). F: digital zoom of the area indicated on image D. G: intensity plot to show staining intensity along the line shown in E. H: representative example of a fixed, isolated guinea pig atrial myocyte co-immunolabeled for IP3R2 (red) and AC1 (green). I: digital zoom of the area indicated on image G. J: intensity plot to show staining intensity along the line shown in H. IP3R1, inositol trisphosphate receptor type 1; IP3R2, inositol trisphosphate receptor type 2; IP3R3, inositol trisphosphate receptor type 3.
Similar to previously published work in guinea pig SAN (20) and atrial myocytes (20, 22) and murine SAN (21), immunolocalization of AC8 indicated a band at or just beneath the sarcolemma. Pixel-by-pixel analysis revealed substantial colocalization between AC8 and IP3R2 in isolated guinea pig atrial myocytes, Pearson’s overlap coefficient R = 0.81 ± 0.02 (n = 14 cells), representative cell shown in Fig. 1, E–G.
AC1 staining was localized to a band which was consistently nearby but predominantly on the intracellular side of IP3R2 staining and signals were not substantially overlapping (R = 0.48 ± 0.05, n = 5, representative cell shown in Fig. 1, H–J). The pattern of AC1 and AC8 expression observed matched that for both AC1 and AC8 described previously in SAN cells (20).
The Effect of IP3 on Cellular Ca2+ Transients Requires Functional Adenylyl Cyclases and PKA
IP3 is not cell permeant and is broken down rapidly within cells. In addition, as activation of α-ARs (e.g., using PE) may result in signaling via alternative pathways including activation of PKC via diacylglycerol (DAG) (39), for our experiments, we used a cell-permeant, caged version of the compound (IP3/PM) to provide cell stimulation specifically via this second messenger from an exogenous source. This IP3 compound crosses the cell membrane, is de-esterified by constitutive esterase activity and trapped, and finally can be activated by “uncaging” through brief exposure to UV light. Exposure of cells to UV light alone under the conditions of this experiment did not affect calcium transient amplitude (Fig. 2B) or shape.
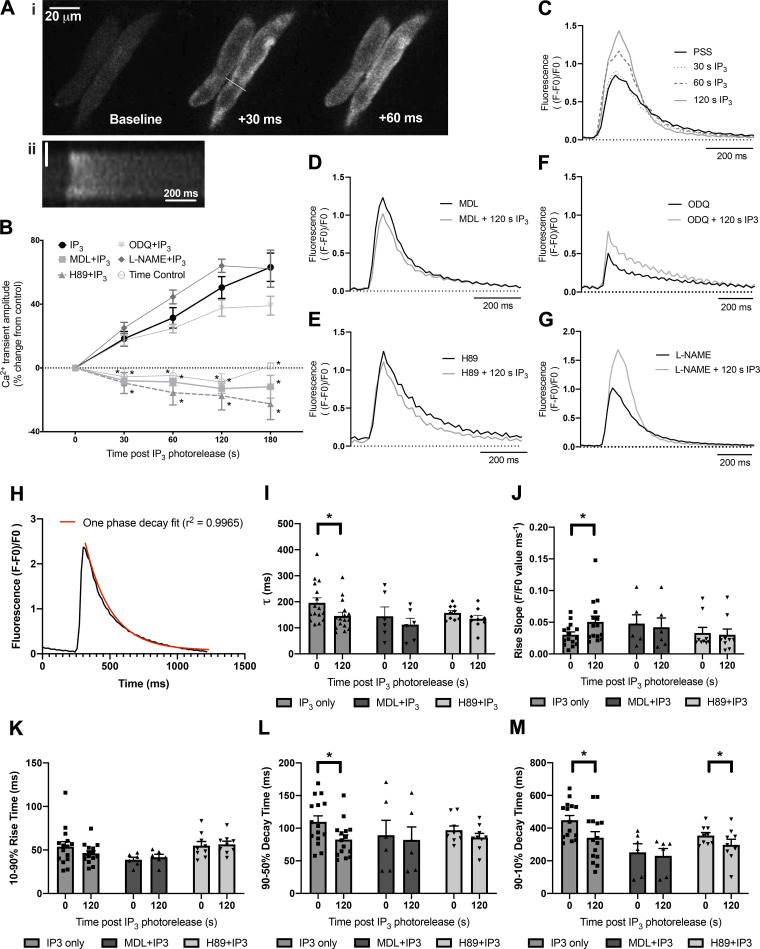
Figure 2.
The direct actions of IP3 in guinea pig atrial myocytes require adenylyl cyclases and PKA. Ai: montage to show the upstroke of a control Ca2+ transient in PSS illustrating the classical U-shaped activation pattern. Numbers indicate time in ms from start of calcium transient upstroke. Aii: pseudolinescan image generated across the line drawn in Ai to show the U-shaped calcium transient seen in guinea pig atrial myocytes. Vertical scale bar indicates 5 µm. B: summary data to show cellular responses to IP3 photorelease (0.5 µmol/L caged-IP3/PM loaded for 1 h) in isolated guinea pig atrial myocytes under control conditions (n = 16) and in the presence of 1 µmol/L H89 (n = 9) to inhibit PKA, 3 µmol/L MDL (n = 6) to inhibit adenylyl cyclases, 10 µmol/L ODQ (n = 10) to inhibit soluble guanylyl cyclase, and 100 µmol/L L-NAME (n = 4) to inhibit nitric oxide synthase. *significant difference in comparison to IP3 photorelease alone (P < 0.05, ANOVA with Dunnett’s post hoc test). C: representative Ca2+ transient response to photorelease of IP3 in PSS under control conditions and at indicated timepoints after photorelease. D–G: representative Ca2+ transients under control conditions and 120 s after photorelease of IP3 in MDL (D), H89 (E), ODQ (F), and L-NAME (G). H: representative Ca2+ trace showing transient decay period fitted using one phase decay equation. I–M: comparison of time constant of decay (τ) (I), maximum rise slope (J), 10%–90% rise time (K), 90-50% decay time (L), and 90-10% decay time (M) for all conditions. Data plotted as means ± SE, *P < 0.05. IP3, inositol trisphosphate; IP3/PM, membrane-permeant caged IP3; PSS, physiological salt solution.
Guinea pig atrial myocytes exhibited the classical “U-shaped” activation pattern (40) of atrial myocyte Ca2+ transients (Fig. 2A, i and ii). Photorelease of IP3 in isolated cardiac atrial myocytes led to a gradual increase in stimulated Ca2+ transient amplitude (e.g., 31 ± 6% increase 60 s after photorelease, n = 16, Fig. 2, B and C. This response was completely abolished in the presence of either the adenylyl cyclase inhibitor MDL (3 µmol/L, n = 6, Fig. 2, B and D) or PKA inhibitor H89 (1 µmol/L, n = 9, Fig. 2, B and E), e.g., change in Ca2+ transient at 60 s after photorelease of −9 ± 2% in the presence of MDL and −16 ± 8% in the presence of H89. The control IP3 response was significantly greater than that of MDL or H89 at all measured timepoints after IP3 photorelease (P < 0.0002 for all comparisons except 30 s after photorelease in H89 where P = 0.0235, ANOVA with Tukey’s test for multiple comparisons), whereas the responses seen in the presence of MDL or H89 were not significantly different from one another, or time controls, throughout all timepoints (P > 0.85 for all comparisons).
As explained in the previous paragraph, our simple hypothesis is that the effects of photoreleased IP3 to increase CaT amplitude results from Ca2+ release from the SR which then activates Ca2+-stimulated adenylyl cyclases that are located nearby. However, a more complex hypothesis deserves consideration since PE responses in cat atrial myocytes have been reported to be dependent on nitric oxide-modulated soluble guanylyl cyclase activity by a mechanism that involves stimulation of NO synthase (eNOS) by IP3-mediated Ca2+ release from the SR (41). We therefore carried out IP3 photorelease in the presence of either 10 µmol/L ODQ to inhibit soluble guanylyl cyclase, or 100 µmol/L L-NAME to inhibit nitric oxide synthase (Fig. 2, B, F, and G). There was no change in the response to IP3 photorelease in the presence of ODQ (P > 0.56 for all timepoints, ANOVA, n = 10, Fig. 2, B and F) or L-NAME (P > 0.45 for all timepoints, ANOVA, n = 4, Fig. 2, B and G); under both conditions, Ca2+ transient amplitude increased significantly over time, beginning rapidly after photorelease of IP3 and was not significantly different to control at any timepoint (Fig. 2B). It therefore appears that under the conditions of our experiments, there is little or no contribution of the nitric oxide-stimulated guanylyl cyclase pathway to the effects of photoreleased IP3.
Further analysis of calcium transient characteristics at the 0 and 120 s timepoints (Fig. 2, H–M and Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.12333305) showed that photorelease of IP3 led to a significant increase in maximum upstroke velocity without affecting 10%–90% rise time (Fig. 2J) and a significant reduction in time constant of decay (Fig. 2I) as well as time to 90% and 50% recovery (Fig. 2, L and M). These changes were no longer seen in the presence of MDL. In the presence of H89, photorelease of IP3 had no significant effect on maximum upstroke velocity or time to 50% recovery, however time to 90% recovery was significantly reduced, though to a lesser extent than the effects seen after exposure to IP3 under control conditions (control; P < 0.0001; H89; P = 0.04).
Separate experiments were performed using external application of PE (10 µmol/L) to determine the effect of inhibition of adenylyl cyclases or PKA where alternative signaling pathways (e.g., PKC, DAG) may be involved (Supplemental Fig. S2). PE caused a 39 ± 10% increase in calcium transient amplitude (n = 6, P = 0.0006 PE condition vs. paired PSS control). As expected, PE no longer caused a significant increase in calcium transient amplitude when applied during inhibition of IP3Rs or α1-adrenergic receptors using 2-APB or prazosin, respectively (P = 0.961 for 2-APB and P = 0.998 prazosin condition vs. drug + PE condition, two-way ANOVA). In a separate set of experiments, PE caused a 35 ± 9% increase in peak-stimulated Ca2+ transient amplitude (n = 8). In the presence of adenylyl cyclase inhibitor MDL (10 µmol/L, n = 5) or PKA inhibitor H89 (1 µmol/L, n = 4), 10 µmol/L PE was no longer able to significantly enhance calcium transient amplitude (P = 0.689 for MDL and P = 0.137 for H89 condition vs. drug + PE condition, two-way ANOVA, Supplemental Fig. S2). This is consistent with the findings of the Blatter group working in cat atrial myocytes (41).
In assessing the importance of the above observations, it is necessary to take into account the influence of ongoing activity of adenylyl cyclases in the absence of stimulation by IP3-mediated Ca2+ release from the SR [described in Refs. (20) and (22)], as well as possible effects of cAMP on the IP3-evoked Ca2+ release (18, 42), and these points are considered in more detail in the discussion. Both MDL and H89 alone were previously shown (22) to reduce the amplitude and slow the time course of CaTs [MDL reduced CaT amplitude by 48 ± 8% (P < 0.001, n = 7), increased the time to peak by 45 ± 5% (P < 0.001, n = 7) and increased the time to 50% decay by 37 ± 13% (P < 0.05, n = 7), whereas H89 reduced CaT amplitude by 37 ± 5% (P < 0.01, n = 6), increased the time to peak by 19 ± 3% (P < 0.001, n = 6) and increased the time to 50% decay by 20 ± 6% (P < 0.05, n = 6)]. However, although CaT amplitude was reduced by both MDL and H89 because of ongoing adenylyl cyclase activity under resting conditions, there was still a substantial remaining CaT (greater than 50% peak amplitude), and if there were important effects of IP3-stimulated Ca2+ release from the SR that did not depend on adenylyl cyclases or downstream PKA effects then these would still be expected to operate and lead to observable increases in CaT amplitude under these conditions. The complete lack of any detectable increase in CaT amplitude following photorelease of IP3 in the presence of MDL or H89 therefore provides a clear indication for the requirement of adenylyl cyclase activity and downstream PKA to bring about the effects of IP3 in the absence of drugs. In a similar way, although some reduction of IP3-evoked Ca2+ release in the presence of MDL and PKA, as suggested from the work of Colin Taylor (18), cannot be excluded, the Ca2+ sensitivity of IP3Rs to IP3 would not be expected to be reduced to zero in the presence of MDL or H89, and therefore again the complete lack of detectable effects of photoreleased IP3 in the presence of MDL or H89 are attributable to the essential requirement of adenylyl cyclase activity and downstream PKA actions for the effects of IP3 in the absence of drugs.
The Positive Chronotropic Effect of PE on the Sinoatrial Node Also Requires Functional Adenylyl Cyclases
It has been established that endogenous generation, or exogenous administration, of IP3 in the SAN leads to an increase in spontaneous beating rate, accompanied by an increase in Ca2+ transient amplitude (7), whereas cAMP from Ca2+-stimulated adenylyl cyclases has been shown to modulate murine heart rate (21) and, specifically, I(f) in these cells (20). Spontaneously beating atrial tissue preparations can also provide a measure of sinoatrial node activity through measurement of beating rate. IP3R2 (7) and AC8 (21) expression have previously been demonstrated in murine sinoatrial node, with that of AC8 highly similar to data presented in Fig. 1 and that of IP3R2 including both peripheral staining as seen in atrial myocytes (e.g., Fig. 1) and separate bands on non-junctional SR. Our own observations (20) demonstrate that AC1 and AC8 appear to show similar distribution within SAN cells as atrial cells. Dose-response curves to PE in the concentration range 0.1–30 µmol/L were carried out on spontaneously beating isolated murine right atria in the presence of 1 µmol/L metoprolol to ensure no confounding action of β-adrenergic receptors and fit with Log(agonist) versus response curves (three-parameter model) by nonlinear regression using a least squares method (Prism 8). Preparations were allowed to reach a stable beating rate in PSS and cumulative addition of PE then took place after either 30 min of metoprolol exposure (used as a time-control for the effect of other inhibitors) or exposure to metoprolol plus named inhibitor. Under these conditions, the positive chronotropic response to PE fits a standard agonist dose-response curve with an EC50 of 0.91 µmol/L [95% confidence interval (CI) 0.68–1.21] and a maximum rate increase of 15.1 ± 0.2% (n = 10, Fig. 3A).
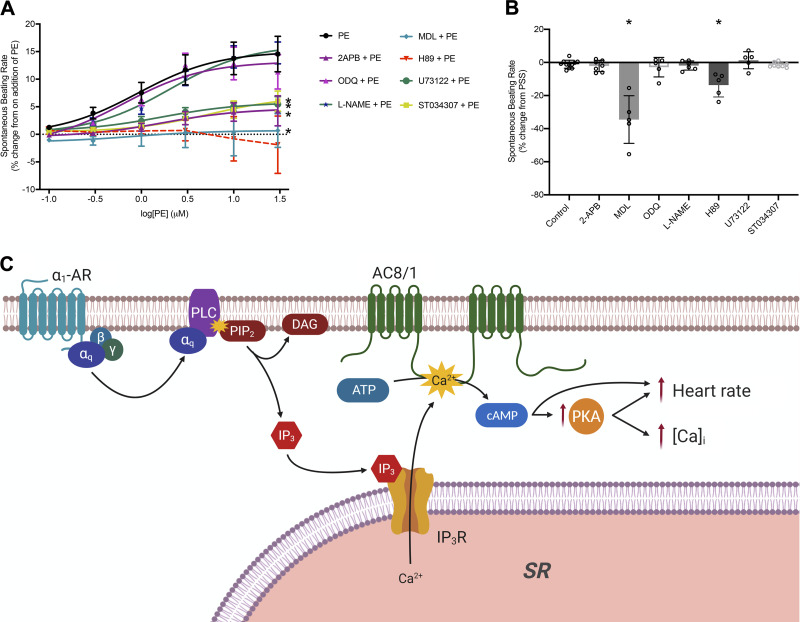
Figure 3.
The positive chronotropic effect of PE requires function of adenylyl cyclases and a proposed scheme for regulation of intracellular calcium via IP3 signaling. A: dose-response curves to show the change in beating rate on cumulative addition of PE to spontaneously beating murine right atrial preparations under control conditions (n = 10) and in the presence of either 2-APB (IP3R inhibitor, 2.5 µmol/L, n = 7), MDL (AC inhibitor, 1 µmol/L, n = 5), ST034307 (AC1 inhibitor, 1 µmol/L, n = 8), H89 (PKA inhibitor, 1 µmol/L, n = 5), U73122 (PLC inhibitor, 5 µmol/L, n = 5), L-NAME (NOS inhibitor, 100 µmol/L, n = 6), or ODQ (sGC inhibitor, 30 µmol/L, n = 5). Dose-response curves (solid lines) were fit with log(agonist) versus response (three-parameter model) using GraphPad Prism 8. *significant reduction in maximum response of the fitted curve by ANOVA in comparison to PE. B: comparison of beating rate change in spontaneously beating murine atrial preparations from stable beating in PSS on addition of the inhibitors used in A, prior to stimulation by PE. Control indicates addition of β-blockade only as a time control (1 µmol/L metoprolol, n = 10). Other conditions are β-blockade plus indicated inhibitor. *P < 0.001 compared to control (one-way ANOVA with Dunnett’s post hoc correction). Data plotted as means ± SE. C: scheme indicates potential mechanisms by which activation of α1 adrenergic receptors (α1-AR) by PE may lead to increased atrial cytoplasmic calcium transients (indicated by [Ca2+]i) and sinoatrial node beating rate based on published observations in addition to our present results. Activation of α1-AR leads to elevated IP3 resulting from cleavage of PIP2 to DAG and IP3 by PLC. IP3 activation of IP3R2 results in release of Ca2+ from the SR, which subsequently leads to activation of Ca2+-sensitive adenylyl cyclases (AC8 or AC1) and activation of PKA by cAMP, or direct effects of cAMP on the funny current I(f). In the proposed scheme, AC8 is placed in the sarcolemma, but it remains to be established whether there is an additional location in the junctional SR and whether nearby AC1 may also be activated by IP3-mediated Ca2+ release. Image created with BioRender. DAG, diacylglycerol; IP3, inositol trisphosphate; IP3R2, inositol trisphosphate receptor type 2; PE, phenylephrine; PSS, physiological salt solution; SR, sarcoplasmic reticulum; 2-APB, 2-aminoethoxydiphenyl borate.
Addition of a low concentration of 2-aminoethyl diphenylborinate (2-APB, 2.5 µmol/L), which is low enough to inhibit IP3-dependent effects in cardiomyocytes without altering cellular Ca2+ transient amplitude or SERCA function (7, 37, 43, 44), had no significant effect on right atrial beating rate over the course of at least 30 min (P = 0.9995, one-way ANOVA with Dunnett’s correction vs. time-control, n = 7, Fig. 3B). In the presence of 2-APB, the maximum rate increase on addition of PE was significantly reduced to 4.7 ± 0.2% (P < 0.0001 vs. PE, ANOVA with Dunnett’s correction, n = 7, Fig. 3A, EC50 1.69 µmol/L, 95% CI 0.99–2.89). In agreement with this, 5 µmol/L U73122 to inhibit IP3 production by inhibition of phospholipase C (PLC) caused no significant change in beating rate (1.3 ± 2.3%, P = 0.9687 vs. time-control, n = 5, Fig. 3B) and significantly reduced the maximum response to PE (to 5.8 ± 0.1%, n = 5, P < 0.0001 vs. PE, ANOVA, Fig. 3A, EC50 1.67 µmol/L, 95% CI 1.19–2.35).
In the presence of 1 µmol/L MDL to inhibit adenylyl cyclase activity, we observed a 34.5 ± 6.4% reduction in beating rate in the absence of further intervention (Fig. 3B, P < 0.0001, one-way ANOVA with Dunnett’s correction vs. time-control, n = 5). Under these conditions, bath application of cumulative doses of PE no longer led to an increase in beating rate (maximum rate change 0.7 ± 0.2%, n = 5, Fig. 3A) without significant effect on EC50 (0.85 µmol/L, 95% CI 0.08–7.63). Application of 1 µmol/L ST034307, a selective inhibitor of AC1 (8), led to a significant reduction in the effect of PE (P < 0.0001 vs. PE, maximum response from dose-response curve fit 7.0 ± 0.3%, n = 8, Fig. 3A) and a significant shift in EC50 (to 5.60 µmol/L, 95% CI 3.73–9.64, P = 0.0163 vs. response to PE, ANOVA with Dunnett’s correction) without a significant change in initial beating rate (Fig. 3B, P = 0.9998). Application of 1 µmol/L H89 to inhibit PKA also led to a significant reduction in beating rate (by 13.7 ± 3.2%, P = 0.0014 vs. time-control, n = 5, Fig. 3B) and completely abolished the effect of PE (largest measured rate change, at 30 µM PE, −1.9 ± 5.2%, n = 5, Fig. 3A). In the presence of H89, it was no longer possible to accurately fit a dose-response curve using the same model, it is therefore excluded from the statistical analyses based on curve fitting.
In agreement with the IP3 photorelease data, neither L-NAME (100 µmol/L, n = 6), nor ODQ (30 µmol/L, n = 5) had a significant effect upon spontaneous beating rate under control conditions, the maximum response to PE or EC50 (Fig. 3, A and B).
DISCUSSION
The present observations indicate the need for an important extension to the proposed signaling pathways underlying the well-recognized actions of IP3 in atria and SAN and provide evidence for an alternative to the previous hypotheses that Ca2+ released from the SR via IP3 receptors may increase the amplitude of Ca2+ transients as a direct result of priming nearby ryanodine receptors (RyRs) (3) or by activating of eNOS located in caveolae in the surface membrane (41). This study represents the first measurements that link direct cellular stimulation with IP3 in atrial myocytes to downstream actions via the generation of cAMP and activation of PKA. Our work is consistent with the hypothesis that interaction of IP3-mediated Ca2+ release with the cAMP system is essential for the positive inotropic and chronotropic effects of this compound in the cardiac atria and sinoatrial node, and that this is physiologically important in the response of these tissues to α-adrenoceptor stimulation. The generation of cAMP by adenylyl cyclases in this newly proposed IP3-dependent pathway will itself have functional effects in SAN by increasing the activation of I(f) (20), and subsequent stimulation of PKA will have well-established downstream effects on phospholamban/SERCA (45), L-type Ca2+ channels (22), and perhaps RyR (46) in both SAN and atria. Structural studies using immunostaining methods (Fig. 1), which initially led us to investigate this intriguing possibility within our preparations, highlight the Ca2+-stimulated isoforms AC8 and AC1 as probable candidates for this interaction.
Before considering subtypes of adenylyl cyclase in more detail, our proposal of a direct involvement of Ca2+-stimulated adenylyl cyclases in the actions of IP3 in atria and SA node should be set in the broader context of interactions between IP3 and cAMP pathways. A particularly interesting possibility is that cAMP influences IP3-evoked Ca2+ release sensitivity to Ca2+ (42). It has been shown that IP3Rs, including IP3R2, can be phosphorylated by PKA enhancing IP3-evoked Ca2+ release (18, 42). In addition, high concentrations of cAMP can cause PKA-independent modulation of IP3Rs (18, 42). We cannot exclude the possibility that such mechanisms also operate in atria and SA node under the conditions of our experiments, but as set out in the results, we do not believe that such a possibility invalidates our use of MDL and H89 to investigate the requirement of adenylyl cyclases for the actions of IP3 in these tissues. We argue that substantial (greater than 50% peak amplitude) CaTs remain in the presence of MDL and H89, and even if the IP3-evoked Ca2+ release were to be reduced in the presence of MDL or H89, this IP3-evoked Ca2+ release would not be expected to be reduced to zero. The suggestion that IP3 receptors remain functional in the absence of cAMP or phosphorylation is based on extensive published work on regulation of IP3R2 receptors, and in all cases, it has been observed that these receptors continue to be activated by IP3 in the absence of cAMP or phosphorylation by PKA (18, 42, 47, 48). In the case of phosphorylation of IP3R2, the extent of enhancement of Ca2+ release has been observed to vary with IP3 concentration, and at 1 µM [see Fig. 2C in (47)], there was no detectable change in the sensitivity of IP3R2 to IP3 following PKA effects. Since no previous work is consistent with the proposal that IP3 receptors are unresponsive to IP3 in the absence of cAMP or without phosphorylation, we argue that our observation of a complete suppression of the effects of IP3 photorelease on CaTs in the presence of MDL and H89 provides convincing evidence supporting the proposal that, at least in atria, IP3-evoked Ca2+ release from the SR activates Ca2+-sensitive adenylyl cyclases and downstream PKA to bring about an enhancement of CaT amplitude. Similar arguments can be advanced for suppression of PE effects on beating rate in SA node, but in this case, additional complications that need to be taken into account are direct effects of cAMP (inhibited by MDL but not H89) and possible roles of DAG that are activated via α receptors in addition to IP3 release, and future experiments will be necessary to further test these possibilities.
Ten mammalian adenylyl cyclase isoforms have been discovered, nine membrane bound and one soluble form. Of these, three are Ca2+ sensitive; AC1 is CaM dependently Ca2+ stimulated (49) with an EC50 for Ca2+ of 75 nmol/L (50), AC8 is CaM dependently stimulated (51, 52) with a Ka for Ca2+ activation of ∼0.5 µmol/L (53) and AC5 is CaM-independently inhibited (54, 55). The majority of previous studies on AC1 and AC8 pertain to roles in the brain, where these enzymes have been implicated in a range of processes including spatial memory formation (56–58), neurodevelopment (59), responses to inflammatory pain (60), and opioid dependence (61). AC1 may also have a role in podocytes of the glomerulus of the kidney (62). Our immunocytochemistry data demonstrate that AC8 is found in close proximity to IP3Rs in cardiac atrial myocytes, whereas AC1 is found in a band just intracellular to these receptors. AC8, therefore, is ideally positioned to transduce local changes in Ca2+ into the cAMP-dependent and PKA-dependent effects detailed in this paper, namely, the modulation of cellular Ca2+ transients in response to IP3. Given the known position of IP3Rs on the junctional SR (3, 5), it is not possible for our staining to distinguish whether AC8 is located on the SR itself or on the surface membrane, situated less than 20 nm away (63, 64). Sucrose-based fractionation of isolated SAN myocytes has indicated that AC1 and AC8 activity is most associated with fractions also containing caveolin-3 (65). In other cell types, AC8 has been localized to caveolae (66), and disruption of lipid rafts has been seen to abolish the stimulation of this cyclase by Ca2+ (53). Taken together, this evidence is consistent with a surface membrane distribution of this enzyme. Although it seems most likely that Ca2+ released via IP3Rs activates colocalized AC8, the selective AC1 inhibitor ST034307 (38) significantly attenuated the response of whole atria beating rate to PE (Fig. 3A), raising the possibility that this Ca2+ could also activate nearby AC1.
The schematic shown in Fig. 3C provides an illustration of the cellular pathway, supported by the data presented in this paper, which may result following activation of the IP3 signaling cascade. Increased generation of cAMP via Ca2+ activation of AC8 or AC1 may lead to activation of PKA and multiple downstream actions that result in increases in cellular Ca2+ transient amplitude or beating rate at the sinoatrial node. AC1 and AC8 expression has previously been demonstrated in the SAN and these AC isoforms have been implicated in the regulation of SAN pacemaker activity (20, 21). Moen et al. (2019) (21) recently demonstrated that overexpression of AC8 in SAN cells results in an increase in heart rate and decrease in heart rate variability. Furthermore, work from the Terrar group has previously demonstrated the involvement of Ca2+-sensitive ACs in the regulation of I(f) in SAN myocytes (20) as well as L-type Ca2+ current in atrial myocytes (22). Taken together, these previous studies implicate both LTCC and HCN4 as potential targets for regulation via IP3 signaling in the context of the data presented in Figs. 2 and 3. The involvement of other targets for PKA phosphorylation however, including ryanodine receptors (RyRs) (46), phospholamban (PLB) (45), and the Na+/Ca+ exchanger (NCX) (67, 68) cannot yet be discounted. It is possible that the activity of PKA augments regulation by PKC, which has also been well documented at these same target sites and is similarly activated via stimulation of Gq-coupled receptors (24, 69–72). The use of caged-IP3 in this study however rather than stimulation of Gq-coupled receptors (Fig. 2) demonstrates that the effects on cellular Ca2+ observed in the present study can occur via the effects of IP3 signaling specifically and are independent of activation of DAG.
Under the conditions of our study, inhibition of ACs or PKA significantly reduced baseline beating rate in right atrial preparations (Fig. 3B). This is consistent with published data from our group (20, 22) and others (73, 74). Indeed, it has previously been shown that heart rate in AC8 overexpressing mice is significantly higher than in their wild-type counterparts (75). The sinoatrial node has a constitutive level of cAMP, which is significantly greater than that of the ventricle in the absence of adrenergic stimulation (26). How much of this activity is attributable specifically to Ca2+-stimulated ACs is not discernable from our experiments, as selective inhibitors are not currently available for all ACs, although 1 μmol/L ST034307 did not affect baseline rate (Fig. 3). The diastolic cell Ca2+ concentration in SAN myocytes, ∼225 nmol/L (76), is considerably higher than that in ventricular cells. Given that AC1 and AC8 proteins have not been shown to be expressed in ventricular tissue (20), it seems possible that differences in the expression of Ca2+-stimulated AC isoforms could contribute to the differences between resting SA nodal and ventricular cAMP concentration as previously reported (26). Indeed, cAMP synthesis activity is high in SAN myocyte lysates in 1 µmol/L Ca2+ but almost abolished in Ca2+-free solution (65), suggesting Ca2+-stimulated cAMP production may be the dominant mechanism in these cells at rest.
Our data indicate that high constitutive cAMP production in the sinoatrial node cannot be attributed to background IP3R activity. Background activity under the conditions of our experiments appears to be negligible as neither 2-APB, nor U73122 had a significant effect on the spontaneous beating rate of intact right atrial preparations when applied in our organ bath setup. This is in contrast to published work from Ju et al. (2011) (7) and Kapoor et al. (2015) (8), who both report significant reductions in spontaneous Ca2+ transient firing rate in response to 2-APB measured using Ca2+ fluorophores in dissected nodal tissue and isolated sinoatrial node myocytes, respectively.
In atrial myocytes isolated from cat, the effects of PE to enhance ICaL have been reported to occur through inhibition of phosphodiesterase downstream of PI-3K-mediated eNOS activation (41). Although we agree that cAMP and PKA are central to the response of atrial myocytes and the sinoatrial node to PE, and IP3, we did not find evidence that nitric oxide or soluble guanylyl cyclase activity were required under the conditions of our experiments.
It has previously been hypothesized that Ca2+ release through IP3Rs acts to enhance atrial myocyte Ca2+ transients by increasing the local Ca2+ concentration around RyRs and thereby enhancing RyR response to the opening of LTCC (3, 16). Although this has been directly observed in an IP3R overexpression model (77), our data investigating the effect of IP3 photorelease in primary isolated myocytes is consistent with the notion that the functional effects of IP3R-mediated Ca2+ release on RyRs occur downstream of intermediary signals. In particular, it would be expected that recruitment of local RyRs in response to photoreleased IP3 would remain after inhibition of adenylyl cyclases or PKA. We observed no change in Ca2+ transient amplitude over time in response to direct application of IP3 in the presence of MDL or H89. It remains possible that RyR sensitization resulting from localized Ca2+ release via IP3Rs is essential in the period before our measurements take place, or that augmented RyR release could itself contribute to recruitment of adenylyl cyclase and PKA signaling.
The results of this study provide a novel mechanism by which a ubiquitous second messenger pathway contributes to physiological signaling in the heart. The presented hypothesis, however, may also provide interest in the context of cardiac pathology. For instance, Mougenot et al. (2019) (78) have recently demonstrated that overexpression of AC8 accelerates age-related cardiac dysfunction through increased hypertrophy and interstitial fibrosis in transgenic mice. Even in healthy atrial myocytes, IP3R signaling can generate arrhythmogenic Ca2+ waves (3, 5). The IP3 pathway may contribute to spontaneous generation of action potentials in pulmonary vein sleeve cells (79, 80), one of the main sites for AF initiation (81), whereas IP3R is known to be upregulated in atrial myocytes from patients with chronic AF (12) and from animal models of atrial fibrillation (AF) (13). In fact, atrial IP3R expression appears to correlate with markers of atrial dysfunction regardless of diagnosed disease state (12). In contrast, RyR expression becomes downregulated in chronic AF (82) and the organization of RyR clusters is disrupted (83). The relative time course of these expression changes during disease development is unknown. The position and character of the signaling domains presented in this paper remain to be determined in the context of the remodeling associated with AF, though the evidence presented in this paragraph highlights the importance of understanding the cellular mechanisms of the IP3 pathway and role of Ca2+-sensitive ACs in both healthy and diseased cardiomyocytes.
The present paper provides novel information on the signaling pathways responsible for physiological responses to IP3, demonstrating a crucial requirement for cAMP and PKA. We have focused on Ca2+-stimulated ACs as an effector of this interaction. Our new observations provide an added level of complexity to Ca2+ modulation in the atria and sinoatrial node and raise questions concerning the importance of these interacting signaling pathways in atrial fibrillation and related pathology.
GRANTS
This work is supported by Sir Henry Dale Wellcome Trust and Royal Society Fellowship (109371/Z/15/Z; to R.A.B.B.). This project was supported by a British Heart Foundation Project Grant (PG/18/4/33521). R.A.C. is a Post-doctoral Scientist funded by the Wellcome Trust and Royal Society (109371/Z/15/Z). T.P.C. was funded through a British Heart Foundation DPhil studentship (FS/05/121) in the D.A.T. lab. S.J.B. is a Post-doctoral Scientist funded by the British Heart Foundation (PG/18/4/33521). T.A. received funding from the Returners Carers Fund (PI R.A.B.B.), Medical Science Division, University of Oxford, the Nuffield Benefaction for Medicine and the Wellcome Institutional Strategic Support Fund, University of Oxford.
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A.B.B. and D.A.T. conceived and designed research; R.A.C., S.J.B., T.P.C., S.R., and T.A. performed experiments; R.A.C., S.J.B., and T.P.C. analyzed data; R.A.C., S.J.B., M.Z., R.A.B.B., and D.A.T. interpreted results of experiments; R.A.C., S.J.B., and T.P.C. prepared figures; R.A.C., R.A.B.B., and D.A.T. drafted manuscript; R.A.C., S.J.B., T.P.C., S.R., M.Z., R.A.B.B., and D.A.T. edited and revised manuscript; R.A.C., S.J.B., M.Z., R.A.B.B., and D.A.T. approved final version of manuscript.
REFERENCES
- 1.Bers DM Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Terrar DA Calcium signaling in the heart. Adv Exp Med Biol 1131: 395–443, 2020. doi: 10.1007/978-3-030-12457-1_16. [DOI] [PubMed] [Google Scholar]
- 3.Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol 10: 939–942, 2000. doi: 10.1016/S0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312: 315–321, 1984. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, Li WH, Lipp P. The role of inositol 1,4,5-trisphosphate receptors in Ca(2+) signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol 541: 395–409, 2002. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosek TM, Williams MF, Zeigler ST, Godt RE. Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol 250: C807–811, 1986. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- 7.Ju YK, Liu J, Lee BH, Lai D, Woodcock EA, Lei M, Cannell MB, Allen DG. Distribution and functional role of inositol 1,4,5-trisphosphate receptors in mouse sinoatrial node. Circ Res 109: 848–857, 2011. doi: 10.1161/CIRCRESAHA.111.243824. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor N, Tran A, Kang J, Zhang R, Philipson KD, Goldhaber JI. Regulation of calcium clock-mediated pacemaking by inositol-1,4,5-trisphosphate receptors in mouse sinoatrial nodal cells. J Physiol 593: 2649–2663, 2015. doi: 10.1113/JP270082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee SG Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281–312, 2001. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remus TP, Zima AV, Bossuyt J, Bare DJ, Martin JL, Blatter LA, Bers DM, Mignery GA. Biosensors to measure inositol 1,4,5-trisphosphate concentration in living cells with spatiotemporal resolution. J Biol Chem 281: 608–616, 2006. doi: 10.1074/jbc.M509645200. [DOI] [PubMed] [Google Scholar]
- 11.Moravec CS, Reynolds EE, Stewart RW, Bond M. Endothelin is a positive inotropic agent in human and rat heart in vitro. Biochem Biophys Res Commun 159: 14–18, 1989. doi: 10.1016/0006-291X(89)92397-8. [DOI] [PubMed] [Google Scholar]
- 12.Yamada J, Ohkusa T, Nao T, Ueyama T, Yano M, Kobayashi S, Hamano K, Esato K, Matsuzaki M. Up-regulation of inositol 1,4,5 trisphosphate receptor expression in atrial tissue in patients with chronic atrial fibrillation. J Am Coll Cardiol 37: 1111–1119, 2001. doi: 10.1016/S0735-1097(01)01144-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z-H, Zhang H-C, Xu Y, Zhang P, Li X-B, Liu Y-S, Guo J-H. Inositol-1,4,5-trisphosphate and ryanodine-dependent Ca2+ signaling in a chronic dog model of atrial fibrillation. Cardiology 107: 269–276, 2007. doi: 10.1159/000095517. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation 101: 2612–2617, 2000. doi: 10.1161/01.CIR.101.22.2612. [DOI] [PubMed] [Google Scholar]
- 15.Gassanov N, Brandt MC, Michels G, Lindner M, Er F, Hoppe UC. Angiotensin II-induced changes of calcium sparks and ionic currents in human atrial myocytes: potential role for early remodeling in atrial fibrillation. Cell calcium 39: 175–186, 2006. doi: 10.1016/j.ceca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Xie H, Zhu P-H, Hu J, Zhao Q, Wang C-S, Yang C. Enhanced activity of inositol-1,4,5-trisphosphate receptors in atrial myocytes of atrial fibrillation patients. Cardiology 114: 180–191, 2009. doi: 10.1159/000228584. [DOI] [PubMed] [Google Scholar]
- 17.Zaccolo M Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol 85: 693–697, 2006. doi: 10.1016/j.ejcb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Taylor CW Regulation of IP3 receptors by cyclic AMP. Cell calcium 63: 48–52, 2017. doi: 10.1016/j.ceca.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thillaiappan NB, Chavda AP, Tovey SC, Prole DL, Taylor CW. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat Commun 8: 1505, 2017. doi: 10.1038/s41467-017-01644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. J Physiol 582: 1195–1203, 2007. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moen JM, Matt MG, Ramirez C, Tarasov KV, Chakir K, Tarasova YS, Lukyanenko Y, Tsutsui K, Monfredi O, Morrell CH, Tagirova S, Yaniv Y, Huynh T, Pacak K, Ahmet I, Lakatta EG. Overexpression of a neuronal type adenylyl cyclase (type 8) in dinoatrial node markedly impacts heart rate and rhythm. Front Neurosci 13: 615, 2019. doi: 10.3389/fnins.2019.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins TP, Terrar DA. Ca(2+)-stimulated adenylyl cyclases regulate the L-type Ca(2+) current in guinea-pig atrial myocytes. J Physiol 590: 1881–1893, 2012. doi: 10.1113/jphysiol.2011.227066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guellich A, Mehel H, Fischmeister R. Cyclic AMP synthesis and hydrolysis in the normal and failing heart. Pflugers Arch 466: 1163–1175, 2014. doi: 10.1007/s00424-014-1515-1. [DOI] [PubMed] [Google Scholar]
- 24.Kramer BK, Smith TW, Kelly RA. Endothelin and increased contractility in adult rat ventricular myocytes. Role of intracellular alkalosis induced by activation of the protein kinase C-dependent Na(+)-H+ exchanger. Circ Res 68: 269–279, 1991. doi: 10.1161/01.RES.68.1.269. [DOI] [PubMed] [Google Scholar]
- 25.Difrancesco D The contribution of the pacemaker current (if) to generation of spontaneous activity in rabbit sinoatrial node myocytes. J Physiol 434: 23–40, 1991. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou Y-Y, Xiao R-P, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514, 2006. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Sirenko S, Riordon DR, Yang DM, Spurgeon H, Lakatta EG, Vinogradova TM. CaMKII-dependent phosphorylation regulates basal cardiac pacemaker function via modulation of local Ca2+ releases. Am J Physioly Heart and Circ Physiol 311: H532–H544, 2016. doi: 10.1152/ajpheart.00765.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boink GJ, Nearing BD, Shlapakova IN, Duan L, Kryukova Y, Bobkov Y, Tan HL, Cohen IS, Danilo P, Jr., Robinson RB, Verrier RL, Rosen MR. Ca(2+)-stimulated adenylyl cyclase AC1 generates efficient biological pacing as single gene therapy and in combination with HCN2. Circulation 126: 528–536, 2012. doi: 10.1161/CIRCULATIONAHA.111.083584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins TP, Bayliss R, Churchill GC, Galione A, Terrar DA. NAADP influences excitation-contraction coupling by releasing calcium from lysosomes in atrial myocytes. Cell calcium 50: 449–458, 2011. doi: 10.1016/j.ceca.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama T, Furuya A, Monkawa T, Yamamoto-Hino M, Satoh S, Ohmori K, Miyawaki A, Hanai N, Mikoshiba K, Hasegawa M. Monoclonal antibodies distinctively recognizing the subtypes of inositol 1,4,5-trisphosphate receptor: application to the studies on inflammatory cells. FEBS Letters 354: 149–154, 1994. doi: 10.1016/0014-5793(94)01099-4. [DOI] [PubMed] [Google Scholar]
- 31.Sugiyama T, Yamamoto-Hino M, Wasano K, Mikoshiba K, Hasegawa M. Subtype-specific expression patterns of inositol 1,4,5-trisphosphate receptors in rat airway epithelial cells . J Histochem Cytochem 44: 1237–1242, 1996. doi: 10.1177/44.11.8918898. [DOI] [PubMed] [Google Scholar]
- 32.Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell 22: 795–806, 2006. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Hattori M, Suzuki AZ, Higo T, Miyauchi H, Michikawa T, Nakamura T, Inoue T, Mikoshiba K. Distinct roles of inositol 1,4,5-trisphosphate receptor types 1 and 3 in Ca2+ signaling. J Biol Chem 279: 11967–11975, 2004. doi: 10.1074/jbc.M311456200. [DOI] [PubMed] [Google Scholar]
- 34.Salvador JB, Egger M. Obstruction of ventricular Ca2+-dependent arrhythmogenicity by inositol 1,4,5-trisphosphate-triggered sarcoplasmic reticulum Ca2+ release. J Physiol 596: 4323–4340, 2018. doi: 10.1113/JP276319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida K, Aramaki M, Nakazawa M, Yamagishi C, Makino S, Fukuda K, Nakamura T, Takahashi T, Mikoshiba K, Yamagishi H. Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis. PloS One 5: e12500, 2010. doi: 10.1371/journal.pone.0012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolte S, Cordelières, FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232, 2006. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122: 498–505, 1997. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 38.Brust TF, Alongkronrusmee D, Soto-Velasquez M, Baldwin TA, Ye Z, Dai MJ, Dessauer CW, van Rijn RM, Watts VJ. Identification of a selective small-molecule inhibitor of type 1 adenylyl cyclase activity with analgesic properties. Sci Signal 10: eaah5381, 2017. doi: 10.1126/scisignal.aah5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Fernandez G, Cabezudo S, Garcia-Hoz C, Beninca C, Aragay AM, Mayor F, Jr., Ribas C. Gaq signalling: the new and the old. Cell Signal 26: 833–848, 2014. doi: 10.1016/j.cellsig.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Hüser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol 494: 641–651, 1996. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YG, Dedkova EN, Ji X, Blatter LA, Lipsius SL. Phenylephrine acts via IP3-dependent intracellular NO release to stimulate L-type Ca2+ current in cat atrial myocytes . J Physiol 567: 143–157, 2005. doi: 10.1113/jphysiol.2005.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tovey SC, Dedos SG, Taylor EJ, Church JE, Taylor CW. Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates direct sensitization of IP3 receptors by cAMP. J Cell Biol 183: 297–311, 2008. doi: 10.1083/jcb.200803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16: 1145–1150, 2002. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 44.Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL. 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell calcium 34: 97–108, 2003. doi: 10.1016/S0143-4160(03)00026-5. [DOI] [PubMed] [Google Scholar]
- 45.Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem 258: 464–471, 1983. [PubMed] [Google Scholar]
- 46.Takasago T, Imagawa T, Shigekawa M. Phosphorylation of the cardiac ryanodine receptor by cAMP-dependent protein kinase. J Biochem 106: 872–877, 1989. doi: 10.1093/oxfordjournals.jbchem.a122945. [DOI] [PubMed] [Google Scholar]
- 47.Betzenhauser MJ, Fike JL, Wagner LE 2nd, Yule DI. Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937 . J Biol Chem 284: 25116–25125, 2009. doi: 10.1074/jbc.M109.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgess GM, Bird GSJ, Obie JF, Putney JW. The mechanism for synergism between phospholipase-C- and adenylylcyclase-linked hormones in liver. Cyclic AMP-dependent kinase augments inositol trisphosphate-mediated Ca2+ mobilization without increasing the cellular-levels of inositol polyphosphates. J Biol Chem 266: 4772–4781, 1991. [PubMed] [Google Scholar]
- 49.Tang WJ, Krupinski J, Gilman AG. Expression and characterization of calmodulin-activated (type I) adenylylcyclase. J Biol Chem 266: 8595–8603, 1991. [PubMed] [Google Scholar]
- 50.Wu Z, Wong ST, Storms DR. Modification of the calcium and calmodulin sensitivity of the type I adenylyl cyclase by mutagenesis of its calmodulin binding domain. J Biol Chem 268: 23766–23768, 1993. [PubMed] [Google Scholar]
- 51.Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem 269: 12190–12195, 1994. [PubMed] [Google Scholar]
- 52.Simpson RE, Ciruela A, Cooper DM. The role of calmodulin recruitment in Ca2+ stimulation of adenylyl cyclase type 8. J Biol Chem 281: 17379–17389, 2006. doi: 10.1074/jbc.M510992200. [DOI] [PubMed] [Google Scholar]
- 53.Smith KE, Gu C, Fagan KA, Hu B, Cooper DM. Residence of adenylyl cyclase type 8 in caveolae is necessary but not sufficient for regulation by capacitative Ca(2+) entry. J Biol Chem 277: 6025–6031, 2002. doi: 10.1074/jbc.M109615200. [DOI] [PubMed] [Google Scholar]
- 54.Colvin RA, Oibo JA, Allen RA. Calcium inhibition of cardiac adenylyl cyclase. Evidence for two distinct sites of inhibition. Cell calcium 12: 19–27, 1991. doi: 10.1016/0143-4160(91)90081-O. [DOI] [PubMed] [Google Scholar]
- 55.Guillou JL, Nakata H, Cooper DM. Inhibition by calcium of mammalian adenylyl cyclases. J Biol Chem 274: 35539–35545, 1999. doi: 10.1074/jbc.274.50.35539. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci 7: 635–642, 2004. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- 57.Wu ZL, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc Natl Acad Sci USA 92: 220–224, 1995. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang M, Moon C, Chan GC-K, Yang L, Zheng F, Conti AC, Muglia L, Muglia LJ, Storm DR, Wang H. Ca-stimulated type 8 adenylyl cyclase is required for rapid acquisition of novel spatial information and for working/episodic-like memory. J Neurosci 28: 4736–4744, 2008. doi: 10.1523/JNEUROSCI.1177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Susick LL, Lowing JL, Provenzano AM, Hildebrandt CC, Conti AC. Postnatal ethanol exposure simplifies the dendritic morphology of medium spiny neurons independently of adenylyl cyclase 1 and 8 activity in mice. Alcohol Clin Exp Res 38: 1339–1346, 2014. doi: 10.1111/acer.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li S, Lee ML, Bruchas MR, Chan GC, Storm DR, Chavkin C. Calmodulin-stimulated adenylyl cyclase gene deletion affects morphine responses. Mol Pharmacol 70: 1742–1749, 2006. doi: 10.1124/mol.106.025783. [DOI] [PubMed] [Google Scholar]
- 61.Zachariou V, Liu R, LaPlant Q, Xiao G, Renthal W, Chan GC, Storm DR, Aghajanian G, Nestler EJ. Distinct roles of adenylyl cyclases 1 and 8 in opiate dependence: behavioral, electrophysiological, and molecular studies. Biol Psychiatry 63: 1013–1021, 2008. doi: 10.1016/j.biopsych.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao Z, He L, Takemoto M, Jalanko H, Chan GC, Storm DR, Betsholtz C, Tryggvason K, Patrakka J. Glomerular podocytes express type 1 adenylate cyclase: inactivation results in susceptibility to proteinuria. Nephron Exp Nephrol 118: e39–e48, 2011. doi: 10.1159/000320382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schulson MN, Scriven DR, Fletcher P, Moore ED. Couplons in rat atria form distinct subgroups defined by their molecular partners. J Cell Sci 124: 1167–1174, 2011. doi: 10.1242/jcs.080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scriven DR, Asghari P, Moore ED. Microarchitecture of the dyad. Cardiovasc Res 98: 169–176, 2013. doi: 10.1093/cvr/cvt025. [DOI] [PubMed] [Google Scholar]
- 65.Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, Vinogradova TM, Lukyanenko YO, Li Y, Ruknudin AM, Boheler KR, van Eyk J, Lakatta EG. Ca(2+) -stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells . J Biol Chem 283: 14461–14468, 2008. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crossthwaite AJ, Seebacher T, Masada N, Ciruela A, Dufraux K, Schultz JE, Cooper DM. The cytosolic domains of Ca2+-sensitive adenylyl cyclases dictate their targeting to plasma membrane lipid rafts. J Biol Chem 280: 6380–6391, 2005. doi: 10.1074/jbc.M411987200. [DOI] [PubMed] [Google Scholar]
- 67.Perchenet L, Hinde AK, Patel KC, Hancox JC, Levi AJ. Stimulation of Na/Ca exchange by the beta-adrenergic/protein kinase A pathway in guinea-pig ventricular myocytes at 37 degrees C. Pflugers Arch 439: 822–828, 2000. doi: 10.1007/s004249900218. [DOI] [PubMed] [Google Scholar]
- 68.Zhang YH, Hancox JC. Regulation of cardiac Na+-Ca2+ exchanger activity by protein kinase phosphorylation–still a paradox? Cell calcium 45: 1–10, 2009. doi: 10.1016/j.ceca.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 69.He JQ, Pi Y, Walker JW, Kamp TJ. Endothelin-1 and photoreleased diacylglycerol increase L-type Ca2+ current by activation of protein kinase C in rat ventricular myocytes. J Physiol 524: 807–820, 2000. doi: 10.1111/j.1469-7793.2000.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woo SH, Lee CO. Effects of endothelin-1 on Ca2+ signaling in guinea-pig ventricular myocytes: role of protein kinase C. J Mol Cell Cardiol 31: 631–643, 1999. doi: 10.1006/jmcc.1998.0899. [DOI] [PubMed] [Google Scholar]
- 71.Yang HT, Sakurai K, Sugawara H, Watanabe T, Norota I, Endoh M. Role of Na+/Ca2+ exchange in endothelin-1-induced increases in Ca2+ transient and contractility in rabbit ventricular myocytes: pharmacological analysis with KB-R7943. Br J Pharmacol 126: 1785–1795, 1999. doi: 10.1038/sj.bjp.0702454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang YH, James AF, Hancox JC. Regulation by endothelin-1 of Na+-Ca2+ exchange current (I(NaCa)) from guinea-pig isolated ventricular myocytes. Cell calcium 30: 351–360, 2001. doi: 10.1054/ceca.2001.0244. [DOI] [PubMed] [Google Scholar]
- 73.Vinogradova TM, Sirenko S, Lukyanenko YO, Yang D, Tarasov KV, Lyashkov AE, Varghese NJ, Li Y, Chakir K, Ziman B, Lakatta EG. Basal spontaneous firing of rabbit sinoatrial node cells is regulated by dual activation of PDEs (phosphodiesterases) 3 and 4. Circ Arrhythm Electrophysiol 11: e005896, 2018. doi: 10.1161/CIRCEP.117.005896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, Yang D, Ruknudin AM, Spurgeon H, Lakatta EG. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circulation research 102: 761–769, 2008. doi: 10.1161/CIRCRESAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- 75.Georget M, Mateo P, Vandecasteele G, Jurevicius J, Lipskaia L, Defer N, Hanoune J, Hoerter J, Fischmeister R. Augmentation of cardiac contractility with no change in L-type Ca2+ current in transgenic mice with a cardiac-directed expression of the human adenylyl cyclase type 8 (AC8. ). FASEB J 16: 1636–1638, 2002. doi: 10.1096/fj.02-0292fje. [DOI] [PubMed] [Google Scholar]
- 76.Sanders L, Rakovic S, Lowe M, Mattick PA, Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol 571: 639–649, 2006. doi: 10.1113/jphysiol.2005.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wullschleger M, Blanch J, Egger M. Functional local crosstalk of inositol 1,4,5-trisphosphate receptor- and ryanodine receptor-dependent Ca2+ release in atrial cardiomyocytes. Cardiovasc Res 113: 542–552, 2017. doi: 10.1093/cvr/cvx020. [DOI] [PubMed] [Google Scholar]
- 78.Mougenot N, Mika D, Czibik G, Marcos E, Abid S, Houssaini A, Vallin B, Guellich A, Mehel H, Sawaki D, Vandecasteele G, Fischmeister R, Hajjar RJ, Dubois-Rande JL, Limon I, Adnot S, Derumeaux G, Lipskaia L. Cardiac adenylyl cyclase overexpression precipitates and aggravates age-related myocardial dysfunction. Cardiovasc Res 115: 1778–1790, 2019. doi: 10.1093/cvr/cvy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Logantha SJ, Cruickshank SF, Rowan EG, Drummond RM. Spontaneous and electrically evoked Ca2+ transients in cardiomyocytes of the rat pulmonary vein. Cell calcium 48: 150–160, 2010. doi: 10.1016/j.ceca.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka Y, Obata K, Ohmori T, Ishiwata K, Abe M, Hamaguchi S, Namekata I, Tanaka H. Angiotensin II induces automatic activity of the isolated guinea pig pulmonary vein myocardium through activation of the IP3 receptor and the Na+-Ca2+ exchanger. Int J Mol Sci 20: 1768, 2019. doi: 10.3390/ijms20071768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. . Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339: 659–666, 1998. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 82.Ohkusa T, Ueyama T, Yamada J, Yano M, Fujumura Y, Esato K, Matsuzaki M. Alterations in cardiac sarcoplasmic reticulum Ca2+ regulatory proteins in the atrial tissue of patients with chronic atrial fibrillation. J Am Coll Cardiol 34: 255–263, 1999. doi: 10.1016/S0735-1097(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 83.Macquaide N, Tuan HT, Hotta J, Sempels W, Lenaerts I, Holemans P, Hofkens J, Jafri MS, Willems R, Sipido KR. Ryanodine receptor cluster fragmentation and redistribution in persistent atrial fibrillation enhance calcium release. Cardiovasc Res 108: 387–398, 2015. doi: 10.1093/cvr/cvv231. [DOI] [PMC free article] [PubMed] [Google Scholar]