Abstract
Women are more likely to suffer from stress-related affective disorders than men, but the underlying mechanisms of sex differences remain unclear. Previous works show that microRNA (miRNA) profiles are altered in stressed animals and patients with depression and anxiety disorders. In this study, we investigated how miRNA expression in the anterior bed nucleus of stria terminalis (BNST) was affected by social defeat stress in female and male California mice (Peromyscus californicus). We performed sequencing to identify miRNA transcripts in the whole brain and anterior BNST followed by qPCR analysis to compare miRNA expression between control and stressed animals. The results showed that social defeat stress induced sex-specific miRNA expression changes in the anterior BNST. Let-7a, let-7f and miR-181a-5p were upregulated in stressed female but not male mice. Our study provided evidence that social stress produces distinct molecular responses in the BNST of males and females.
Keywords: Sex differences, extended amygdala, social stress, miRNA, let-7, depression, anxiety
1. Introduction
Sex differences are an important aspect of stress-related affective disorders. Women are at significantly higher risk than men to suffer from depression and anxiety disorders [1-4]. However, there are still large gaps in our understanding of the neurobiological mechanisms of increased female vulnerability. Human postmortem research has reported sex differences in gene expression of depressive patients indicating sex-specific gene regulation [5-10]. Growing evidence suggests that altered microRNA (miRNA) function might contribute to the pathology of psychiatric diseases, which provides us with a new perspective to investigate the underlying mechanisms of sex differences in stress-related disorders on a molecular level [11-16].
MicroRNAs are a class of small non-coding RNAs that negatively regulate posttranscriptional gene expression [17,18], and are often differentially expressed between males and females [19,20]. MicroRNAs play a critical role in normal cellular function and have been proposed as potential biomarkers or therapeutic targets for affective disorders[21-26]. Clinical studies have shown that miRNA profiles are altered in the blood of patients with depression [27-29] and anxiety disorders [30,31], but less is known about their functions within the brain. In rodent studies, exposure to chronic stress leads to differential expression of miRNA transcripts in different regions of the limbic system, including the nucleus accumbens [32,33], amygdala [34-36], and prefrontal cortex [37-39]. In one study, social isolation stress induced sex-specific changes in miRNA expression in the bed nucleus of stria terminalis (BNST)[40].
The BNST, also known as the extended amygdala, is a sexually dimorphic brain region involved in modulating anxiety in both human and rodent studies [41-46]. Our group has previously demonstrated that stress-induced sex differences in brain-derived neurotrophic factor (BDNF) protein expression contributed to sex-specific behavioral phenotypes [47]. Social defeat stress increased BDNF protein expression in the anterior BNST of female but not male California mice (Peromyscus californicus) and increased BDNF activity in stressed females facilitated increased social avoidance behavior. Despite a significant increase in protein expression in stressed females, there were no sex differences in Bdnf mRNA expression. These findings suggest the possibility of distinct post-transcriptional regulation processes in female and male mice in response to social defeat.
The increase of BDNF protein expression in stressed females could result from a decrease of its upstream miRNA suppressors. As a bidirectional mediator of miRNA functions, BDNF is not only modulated by miRNAs [48,49] but also serves as an upstream regulator of a variety of miRNAs transcripts [50,51]. For example, BDNF upregulates Lin28, which is an RNA binding protein that further suppresses the biogenesis of the let-7 miRNA family[50,52-55]. Let-7 is one of the most abundantly expressed miRNA families in the brain [56] and is altered in both depressed patients [27,57,58] and animals exposed to chronic and acute stress [59]. In this study, we examined potential BDNF upstream suppressors (miR-15b-5p, miR-181a-5p), BDNF downstream targets (Lin28 and let-7 family members), and other abundant transcripts in the BNST (miR-340-5p and miR324-3p) in California mice (Peromyscus californicus). In this species, both male and female mice aggressively attack the intruders to defend their territories in the wild [60] as well as in lab settings [61,62]. However, social defeat produces a more significant decrease in social interaction behavior in female mice [63]. Understanding the underpinning of such behavioral discrepancy might give us insights on sex differences in stress-related disorders.
Since the fully sequenced genome was not available for this species at the onset of the study, we first performed sequencing to obtain a miRNA transcriptome in the whole brain and in punch samples of the whole BNST. Once these sequences were known, we used qPCR analysis to compare miRNA expression in the anterior BNST between control and stressed California mice. We hypothesized that following social defeat, 1) female and male mice would exhibit distinct miRNA differential expression patterns and 2) stressed female, but not male mice, would show a decrease in BDNF suppressor expression, an increase in Lin28 and a decrease in let-7 expression.
2. Materials and Methods
2.1. Animals
Adult female and male California mice (P. californicus) between 3 to 6 months old from our laboratory colony were co-housed in same-sex groups of 2 to 3. Mice were kept on a 16:8 Light:Dark cycle, a summer-like light cycle commonly used in Peromyscus studies [64,65], and fed ad libitum. All experiments were performed under dim red light in accordance with and approval of the Institutional Animal Care and Use Committee at the University of California, Davis. Estrous cycles were monitored via post-mortem vaginal lavage.
2.2. Sample Collection for MicroRNA Transcriptome
To acquire the California mouse miRNA transcriptome, whole brain RNA samples were obtained. Three females and three males were anesthetized with isoflurane and decapitated. Whole brains were flash frozen on dry ice. Each brain was bisected at the midline and homogenized in 3mL of Trizol. RNA was then extracted by using the mirVANA miRNA Isolation Kits (Ambion™ AM1560) following the manufacturer’s instructions. These samples were sequenced to produce a miRNA brain transcriptome for the California mouse. To determine which of these transcripts were present in the BNST, we extracted RNA from punch samples comprising of both anterior and posterior BNST (whole BNST). Samples were acquired from 12 mice in total, 3 for each group: control male, control female, stressed male and stressed female. Two weeks following stress, the mice were anesthetized with isoflurane and decapitated [66]. Previous studies from our group have shown that the behavioral impact of social defeat increases over time and there is a significant sex x stress interaction two weeks following the last episode of defeat [63]. Brains were rapidly removed, and 2 mm slices were dissected using a brain matrix (Trainor et al. 2003). The posterior side of the slice was at approximately −0.24 bregma and the anterior side was at approximately 0.75 bregma. These coordinates were used based on the California mouse brain atlas (brainmaps.org). The whole BNST was dissected out using an 1mm inner diameter punch tool to include both the anterior and posterior subregions. Samples were frozen on dry ice and then stored at −40°C until RNA extraction. Punch samples were used for this study due to the large amount of RNA required for miRNA library preparation.
2.3. Social Defeat Stress
Both female and male California mice attack same-sex intruders to defend their territories [60-62]. Animals were first randomly assigned to either control or social defeat. Mice assigned to defeat were placed in a cage of an aggressive same-sex resident until they were attacked by the resident seven times or until seven minutes had elapsed, which normalizes the intensity of aggression between females and males. Residents were between 6 months to 2 years old. All mice were attacked at least once during each defeat session. Control animals were placed in an empty cage for 7 minutes. This process was repeated over the course of 3 days, each day with a new aggressive resident. The total number of attacks received by stressed males (6.1±0.3) and females (5.1±0.5) was not significantly different throughout the 3 defeat sessions (t15=1.63, p=0.12).
2.4. Library Preparation
RNA was extracted from the whole brain samples by using the mirVANA miRNA Isolation Kit (Ambion™ AM1560) following the manufacturer’s instructions and run on a Bioanalyzer. The average RNA Integrity Number (RIN) of the RNA samples used for library prep was 7.55 ± 0.37. One μg of RNA from each sample was prepared for microRNA library identification through the TruSeq Small RNA Library Preparation Kits (Illumina RS-200-0012). Briefly, ligation reactions were used to attach adaptors to RNA samples and reverse transcription was performed using Superscript II (Invitrogen). Eleven cycles of PCR (98 °C for 10s, 60 °C for 30s and 72 °C for 15s) were performed to amplify each library. MicroRNA was then isolated through gel purification. Each library was run out on 8% TBE buffer polyacrylamide gels and a single 145 bp band (containing miRNA sequence and adaptors) was dissected from the gel and then purified. Purified libraries were re-analyzed on the Bioanalyzer to confirm the correct size selection. Libraries were then pooled and sequenced on a single lane on an Illumina HiSeq (paired-end 50 bp reads).
To prepare libraries for the whole BNST, it was necessary to pool samples from 3 mice in order to acquire 1 μg of RNA. Thus, one library was prepared for sequencing from each of four groups (control female, control male, stressed female, stressed male). Each female library contained samples from two females in metestrus and one female in diestrus. A previous study from our lab has shown that estrous cycle stage does not confound the effects of defeat stress on social behaviors in California mice [63]. Libraries were prepared exactly as described above. The four libraries were pooled and sequenced on a single lane of HiSeq (single end 50 bp reads).
2.5. Bioinformatics
All reads were trimmed to remove adapter sequences using Scythe (https://github.com/vsbuffalo/scythe; commit ID c128b19). Paired-end small RNA-Seq reads were then joined using FLASH [67] in order to increase base qualities. Both paired-ended and single-ended reads were trimmed to remove low quality bases using Sickle (https://github.com/najoshi/sickle; commit ID 7667f14). Trimmed reads were used for miRNA identification and (novel miRNA) prediction using miRDeep 2.0.0.5 [68,69], which aligns small RNA reads to a genome sequence, searches for a depth signature associated with miRNA and its breakdown products, and compares miRNA sequences predicted from the signatures to a miRNA sequence database. In this case, reads were aligned to both the Mus musculus genome (UCSC package, Illumina iGenome) and separately to the Peromyscus maniculatus bairdii genome (GenBank GCA_000500345.1). Since there was no annotated California mice genome, mapped reads were annotated by using the miRNA sequences of Mus musculus, Rattus norvegicus, and Cricetulus griseus from miRbase [70]. Novel miRNA sequences from unannotated sequences were based on alignments and RNA folding. Finally, raw abundances of each transcript were provided in the form of aligned read counts. All raw sequence data are available from NCBI under the BioProject accession number PRJNA263194.
For target prediction, we used TargetSpy, an algorithm that uses a machine learning approach to identify miRNA transcripts that target a specific genomic sequence [71]. TargetSpy was used to identify miRNA transcripts California mouse Bdnf mRNA (GenBank:JX977026) using the “sensitive” and “specific” modes to predict target interactions between the miRNA’s identified with miRDeep and the 3’ untranslated region of the California mouse BDNF gene. Annotation of let-7 transcripts was based on previously published sequences in Mus musculus [50].
We also used miRWalk 3.0 [72] to predict gene targets of stress-regulated miRNAs in the BNST in male and female mice, respectively. Transcripts with an arbitrary cutoff of ∣ logfc ∣ >2 were selected for gene target analysis. The Mus musculus database was used and only targets predicted by both the miRWalk and miRDB algorithms with a binding probability of 1 were selected for further gene ontology (GO) analysis. For GO biological process analysis, we used the Enrichr online tool and the results were ranked by p-values (p<0.05) [73,74].
2.6. Quantitative real-time PCR analysis
Seven miRNA transcripts were chosen for real-time PCR analysis in the anterior BNST. The California mouse sequence for each transcript was determined to be an exact match with Mus musculus to allow for the use of TaqMan Gene Expression Assays. We examined the three most abundant let-7 transcripts observed in the whole BNST libraries: let-7a, let-7c, and let-7f. Next, we examined the expression of miR-15b-5p, which was the most abundant miRNA from the TargetSpy analysis for which a Taqman assay was available. Finally, we examined the miR-181a-5p, miR-340-5p, and miR-324 because these transcripts were highly abundant in the whole BNST libraries. We normalized expression to U6, and raw Ct’s were checked to confirm that were no differences among treatment groups (p > 0.4).
In this experiment, female and male mice were randomly assigned to defeat or control condition as previously described (n=8 control males, n=8 stressed males, n=7 control females, n=9 stressed females). Two weeks after defeat, mice were euthanized with isoflurane and decapitated. The brains were quickly removed, flash frozen on dry ice, and stored at −40 °C. Brain were sectioned on a cryostat at −10°C with a thickness of 500 μm and immediately immersed into RNAIater® for approximately 24 hours at 4°C [47]. An 1mm sized punch tool was used to collect bilateral samples from the anterior portion of the bed nucleus of the stria terminalis. Samples were kept frozen at −40°C until RNA extraction.
RNA was extracted from the punch samples by using the mirVANA miRNA Isolation Kit as described above. Samples were diluted to a maximum concentration of 10 ng/μL before reverse transcription by using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems™ 4366596). The miRNA expression levels were detected by using the Taqman® Universal Master Mix II, No AmpErase® UNG (Applied Biosystems™ 4352042) and Applied Biosystems® Custom TaqMan® MGB Probes. The following custom TaqMan® Gene Expression Assays were used for qPCR (Let-7a, #002478; Let-7c, #000379; Lef-7f, #000382; miR-15b-5p, #000390; miR181a-5p, #000480; miR-340-5p, #002258; miR324-3p, #002509). Real-time PCR cycling conditions began at 95°C for 10min, followed by 40 cycles of 95°C for 15s, and 60°C for 60s.
2.7. Statistical analysis
Comparisons of the miRNA expression between control and stressed animals were performed using SPSS software. Non-parametric Mann-Whitney U test was used to test for statistical significance because of non-normal distribution of data.
3. Results
3.1. MicroRNA sequencing
RNAseq analysis of the whole BNST punch samples was used to identify transcripts for further qPCR analysis in male and female mice. TargetSpy analysis of whole brain libraries identified 95 miRNA transcripts expected to bind to the California mouse Bdnf mRNA. All but one of these transcripts were predicted to bind to the 3’ untranslated region (Supplementary Table 1). The two most abundant transcripts (miR-181a-5p and miR-15b-5p) were selected for follow-up qPCR analysis (Supplementary Table 2).
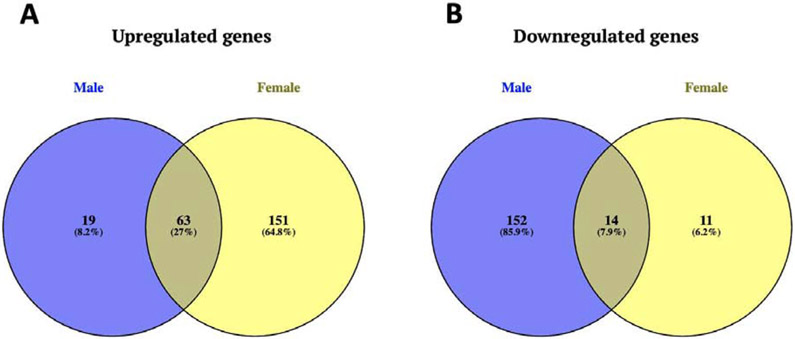
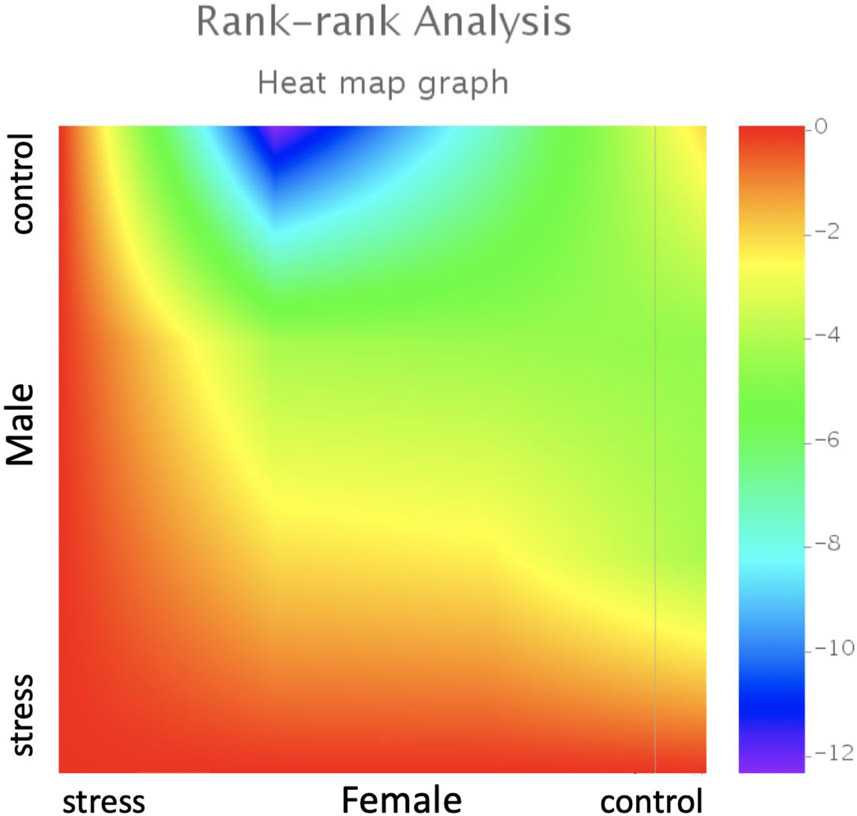
Social defeat had sex-specific effects on miRNA expression in the BNST between male and female mice. In general, there were more transcripts showing a positive fold-change in stressed females (Figure 1A) and more transcripts showed negative fold-change in stressed males (Figure 1B). A Rank-Rank Hypergeometric Overlap (RRHO) plot was used to broadly compare gene expression in the BNST region of male and female mice (Figure 2). RRHO is a threshold free approach that compares multiple gene expression datasets to identify overlapping trends [75,76]. The upper right quadrant shows that control females and males shared similar miRNA expression patterns at baseline, but that social defeat induced divergent transcriptional responses as shown in the lower left quadrant. The plot suggests that distinct miRNA profiles were induced by social defeat in male and female mice. This hypothesis was tested more directly with qPCR. The venn diagrams and RRHO plot do not indicate statistical significance but instead compare the overall trend of miRNA profile changes between male and female mice in response to social stress.
Figure 1: Stressed male and female mice show distinct miRNA differential expression patterns in the whole BNST.
There were 151 uniquely upregulated miRNA transcripts in stressed females comparing to 19 in stressed males and 63 transcripts that were upregulated in both sexes (A). There were 152 uniquely downregulated transcripts in stressed males comparing to 11 in females and only 14 transcripts that were downregulated in both sexes (B).
Figure 2: Threshold-free comparison of miRNA expression using rank-rank hypergeometric overlap analysis.
Pixels represent overlap between the transcriptome of female and male California mice with a color-coded significance of overlap (−log10(p-value)) of a hypergeometric test. Lower left quadrant shows an anti-correlation between miRNA transcript lists, where highly expressed miRNA transcripts in stressed females appear lowly expressing in stressed males. One library per group (male/female, control/stress) was sequenced and each library was prepared from pooled RNA extracted from BNST samples of 3 mice.
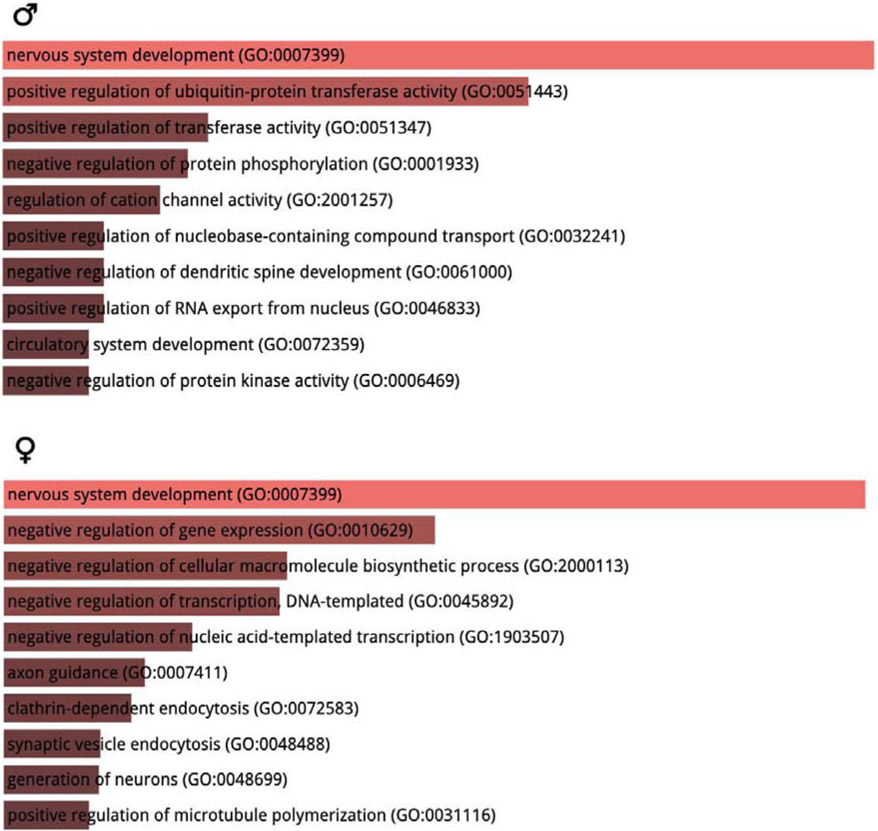
We also used miRWalk 3.0 and Enrichr to predict potential gene targets of stress-regulated miRNA transcripts in the BNST region and performed GO biological process analysis of these predicted targets. The analyses showed that “nervous system development” was the most enriched GO biological process in both sexes but otherwise different processes were expected to be affected by stress in males and females (Figure 3).
Figure 3: Gene ontology (GO) biological processes analysis of stress-regulated miRNAs.
The gene targets of stress regulated miRNAs were predicted by miRWalk 3.0 and the top 10 most eriched biological pathways were predicted by Enrichr. Distinct biolgocial pathways were involved in stressed males versus females.
3.5. qPCR analysis
Based on previous results, we hypothesized that stress-induced increases in BDNF protein could be driven by a decrease in miRNA suppression of the Bdnf mRNA. We predicted that stress would decrease expression of miR-181a-5p and miR-15b-5p. This hypothesis was not supported by our qPCR analysis. Surprisingly, the most abundant transcript in the whole BNST libraries, miR-181a, had increased expression in stressed females (Figure 4A, Mann-Whitney U=42.0, p < 0.05) but was not affected by stress in males. There were no sex differences or effects of stress on miR-15b expression (Figure 4B). The transcripts miR-324 or miR-340 were not identified by Targetspy as potential regulators of Bdnf and showed no differences in expression (Figure 4C, 2D).
Figure 4: Comparisons of BDNF supressor expression between control and stress animals.
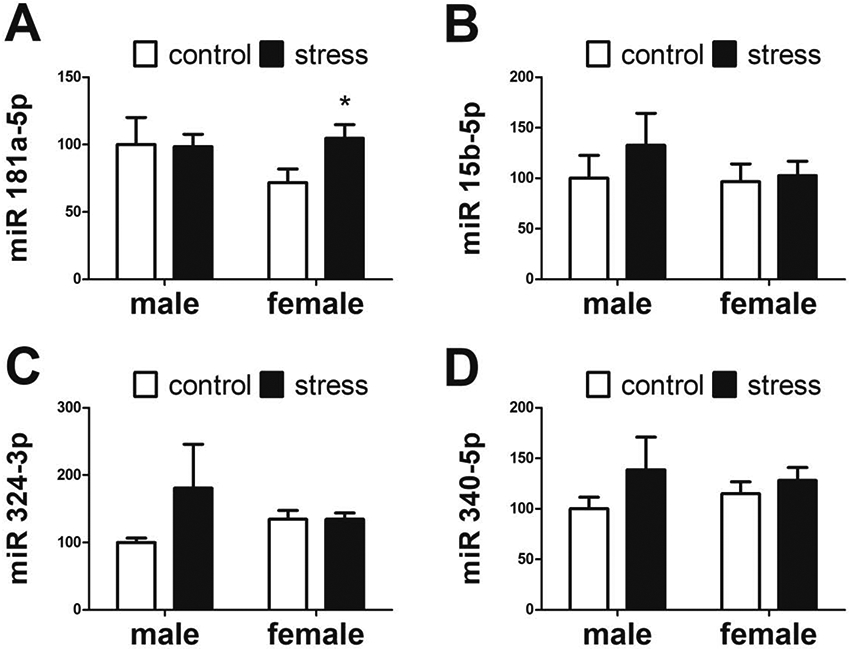
There was a significant increase of miR-181a-5p (A) expression in stressed females (n=9) compared to control females (n=7) but there were no differences between control (n=8) and stressed males (n=8). There were no significant differences in miR-15b-5p (B), miR-324-3p (C), or miR-340-5p (D) expression. All expression data normalized to control males. * p < 0.05 vs same-sex control group.
We then tested the hypothesis that increased BDNF activity in stressed females would increase the expression of Lin28 and decrease the expression of let-7 transcripts. Contrary to our hypothesis, social defeat increased expression of let-7 transcripts in females. Stressed females had increased expression of let-7a (Figure 5A, Mann-Whitney U=39.0, p < 0.05) and let-7f (Figure 5C, Mann-Whitney U=39, p < 0.05) compared to control females. There was a nonsignificant trend for stress to increase expression of let-7c (Figure 5B, Mann-Whitney U=36.0, p = 0.07) in females. No effects of defeat on let-7 expression were observed in males. Although Lin28 expression in stressed females was not significantly different from control females, it was higher in stressed females than stressed males (Figure 5D, Mann-Whitney U, p = 0.05).
Figure 5: Relative expression of BDNF downstream transcripts expression between control and stress animals.
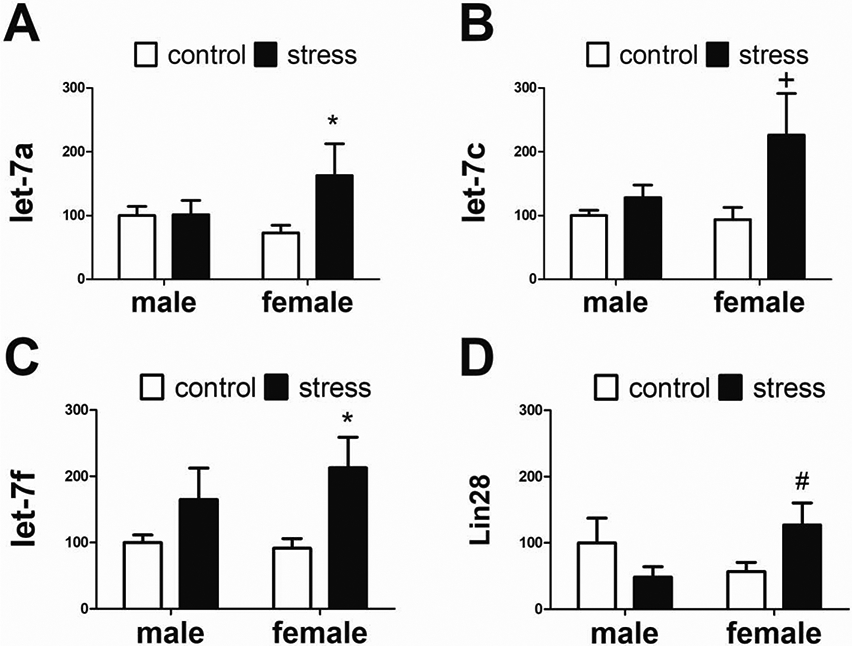
In females, stress (n=9) increased let-7a (A) and let-7f (C) compared to controls (n=7). In males there were no differences between control (n=8) and stressed (n=8) groups. There were nonsignificant trends for stress to increase let-7c and Lin28 expression in females. All expression data normalized to control males. * p < 0.05 vs control female, # p=0.05 vs control male, + p=0.07 vs control female.
4. Discussion
Overall, both RRHO and qPCR analyses provided evidence that social defeat had sex-specific effects on miRNA expression in the whole BNST and the anterior BNST, respectively. This was confirmed with qPCR analysis, where we observed more upregulation of miRNA transcripts in stressed females than in stressed males , which is consistent with the previous work examining the effects of social isolation stress on miRNA expression in the BNST [40]. In addition, the GO analysis revealed that the stress-regulated miRNAs were mostly involved in distinct biological processes between male and female mice. Since the direction of the expression changes was not consistent with our specific hypotheses related to BDNF, other pathways independent from BDNF action might be involved in miRNA regulation. Below, we consider alternative hypotheses that could explain our results.
Contrary to our initial hypothesis, the potential BDNF suppressor miR15b-5p did not change following social defeat in either sex. Since a variety of miRNAs have been reported to suppress BDNF expression in the brain, such as miR-30a-5p [48], miR-124a [77] miR-212 [49] etc., it is very likely that BDNF was also under regulation of other transcripts in the anterior BNST. It is also possible that BDNF protein is synthesized in other brain regions and transported retrogradely[78] or anterogradely [79] to the BNST. It has been previously demonstrated that 2 hours following social defeat, BDNF mRNA and protein expression increases in medial prefrontal cortex, basolateral and central amygdala [80], which could serve as possible sources of BDNF in the BNST [81].
An interesting finding from the qPCR analysis is the increase of let-7a and let-7f expression in stressed female but not male mice. The let-7 family is highly conserved across mammalian species and involved in a wide range of biological processes, including cell development and differentiation [82]. Both let-7a and let-7f expression is downregulated in the periphery of patients with stress-related mood disorders [27,83]. Several rodent studies indicate that let-7a might play an important role in response to both acute and chronic stress. For example, let-7a expression increased in the frontal cortex of CD-1 mice exposed to acute restraint stress [59] as well as the in the central amygdala of rats exposed to both acute and chronic stress [84]. Nevertheless, these studies were done only in male animals. When the impact of subchronic variable stress (SCVS) on gene expression in the nucleus accumbens was studied in both sexes, let-7a and let-7f were upregulated in female but not male C57BL/6J mice [32]. These results parallel effects of SCVS on depressive-like behaviors, which are upregulated in female but not male mice [7]. Similarly, social defeat has stronger effects on social behavior in female California mice versus males [47]. We could speculate that the let-7 family may act across a broad network of brain regions to play an important role in increased female susceptibility to stress.
Let-7a might interact with serotonin receptors in stressed female mice to modulate depressive and anxiety-like behaviors. A previous study demonstrated that rats exposed to maternal deprivation and chronic unpredictable stress showed upregulation of hippocampal let-7a expression and decreased sucrose preference rate compared to control, an indication of anhedonia [85]. In the same study, serotonin 4 receptor (HTR4) protein and mRNA expression were negatively correlated with let-7a expression. The activation of serotonin receptors in the BNST has also been linked to regulation of fear and anxiety [86-88]. However, there is no direct evidence of let-7a suppressing HTR4 expression yet.
Another interesting finding was that miR-181a-5p expression increased in stressed female but not male mice. The increase of miR-181a-5p expression in stressed females is consistent with a former finding that the same transcript was upregulated in female rats exposed to social isolation stress [40]. MiR-181a-5p might play a sex-specific role in stress response. A previous study has demonstrated that miR-181a suppresses AMPA-type glutamate receptor expression in hippocampal neurons [89]. Since infusion of AMPA receptor antagonists into the BNST blocks light-enhanced startle in rats [90], the increase of miR-181a-5p might serve to mitigate anxiety-like behaviors in a non-social context through downregulating AMPA receptors. This could explain why social defeat led to decreased social interaction time in stressed females but had no effects on behaviors in open field test or light-dark box test [63].
The conclusions that could be drawn from the sequencing data were limited due to the need for pooling RNA samples for library preparation. The main goal of the sequencing experiment was to acquire miRNA transcriptome and determine which transcripts were present in the BNST. Recent developments in library preparation methods for miRNA sequencing now require less RNA input [91,92], which would allow for the sequencing of independent biological replicates and produce statistically meaningful data. Another limitation of this study was the lack of behavioral analysis that precluded correlational analysis with individual differences in behavior. However, sex-dependent effects of social defeat in California mice are robust, as analyses of hundreds of mice across different experiments show stronger effects on social approach [93] and social vigilance (Wright et al. unpublished) in females than males. Future studies should combine miRNA measurement in mice that have been behaviorally tested. This would allow for correlational analysis between individual transcripts and specific behaviors, such as social approach or social vigilance [94].
In conclusion, this study showed that social defeat stress induced sex-specific miRNA expression patterns in the anterior BNST, providing additional evidence that female and male mice process social stress differently on a molecular level. Chemical stressors such as exposure to endocrine disrupting chemicals can have similar effects as social stressors [95], and recent data indicate that endocrine disrupting chemicals can induce sex-dependent alterations in miRNA profiles in California mouse limbic system [96]. Future studies that investigate the functional significance of miR181a and the let-7 family members would help us identify sex-specific biomarkers and therapeutic targets for stress-related affective disorders.
Supplementary Material
Highlights.
Social defeat stress induces sex-specific miRNA expression patterns in the BNST.
There is more upregulation of miRNAs in stressed females and more downregulation in males.
Let-7a, let-7f, and miR-181a-5p expression is increased by social defeat stress in female but not male mice.
Acknowledgements
The authors thank C. Clayton for animal care and two anonymous reviewers for constructive comments for revision this manuscript. This work was supported by a UC Davis Academic Senate Grant and R01 MH121829 to BCT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO, Sex Differences in the Prevalence and Detection of Depressive and Anxiety Disorders in General Health Care Settings: Report From the World Health Organization Collaborative Study on Psychological Problems in General Health Care, Arch Gen Psychiatry. 55 (1998) 405 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- [2].Altemus M, Sex differences in depression and anxiety disorders: Potential biological determinants, Hormones and Behavior. 50 (2006) 534–538. 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- [3].Caballo VE, Salazar IC, Irurtia MJ, Arias B, Hofmann SG, Differences in social anxiety between men and women across 18 countries, Personality and Individual Differences. 64 (2014) 35–40. 10.1016/j.paid.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Altemus M, Sarvaiya N, Neill Epperson C, Sex differences in anxiety and depression clinical perspectives, Frontiers in Neuroendocrinology. 35 (2014) 320–330. 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vialou V, Feng J, Robison AJ, Nestler EJ, Epigenetic Mechanisms of Depression and Antidepressant Action, Annu. Rev. Pharmacol. Toxicol 53 (2013) 59–87. 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS, Sex differences in glutamate receptor gene expression in major depression and suicide, Mol Psychiatry. 20 (2015) 1057–1068. 10.1038/mp.2015.91. [DOI] [PubMed] [Google Scholar]
- [7].Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Menard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ, Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress, Journal of Neuroscience. 35 (2015) 16362–16376. 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J, Biological Sex Differences in Depression: A Systematic Review, Biological Research For Nursing. 20 (2018) 383–392. 10.1177/1099800418776082. [DOI] [PubMed] [Google Scholar]
- [9].Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, Logan RW, Tseng G, Lewis DA, Sibille E, Opposite Molecular Signatures of Depression in Men and Women, Biological Psychiatry. 84 (2018) 18–27. 10.1016/j.biopsych.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Issler O, van der Zee YY, Ramakrishnan A, Wang J, Tan C, Loh Y-HE, Purushothaman I, Walker DM, Lorsch ZS, Hamilton PJ, Peña CJ, Flaherty E, Hartley BJ, Torres-Berrío A, Parise EM, Kronman H, Duffy JE, Estill MS, Calipari ES, Labonté B, Neve RL, Tamminga CA, Brennand KJ, Dong Y, Shen L, Nestler EJ, Sex-Specific Role for the Long Non-coding RNA LINC00473 in Depression, Neuron. 106 (2020) 912–926.e5. 10.1016/j.neuron.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hunsberger JG, Austin DR, Chen G, Manji HK, MicroRNAs in Mental Health: From Biological Underpinnings to Potential Therapies, Neuromol Med. 11 (2009) 173–182. 10.1007/s12017-009-8070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Forero DA, van der Ven K, Callaerts P, Del-Favero J, miRNA genes and the brain: implications for psychiatric disordersa, Hum. Mutat 31 (2010) 1195–1204. 10.1002/humu.21344. [DOI] [PubMed] [Google Scholar]
- [13].Xu B, Karayiorgou M, Gogos JA, MicroRNAs in psychiatric and neurodevelopmental disorders, Brain Research. 1338(2010) 78–88. 10.1016/j.brainres.2010.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hammack SE, Cooper MA, Lezak KR, Overlapping neurobiology of learned helplessness and conditioned defeat: Implications for PTSD and mood disorders, Neuropharmacology. 62 (2012) 565–575. 10.1016/j.neuropharm.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Geaghan M, Cairns MJ, MicroRNA and Posttranscriptional Dysregulation in Psychiatry, Biological Psychiatry. 78 (2015) 231–239. 10.1016/j.biopsych.2014.12.009. [DOI] [PubMed] [Google Scholar]
- [16].Issler O, Chen A, Determining the role of microRNAs in psychiatric disorders, Nat Rev Neurosci. 16 (2015) 201–212. 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- [17].Eulalio A, Huntzinger E, Izaurralde E, Getting to the Root of miRNA-Mediated Gene Silencing, Cell. 132 (2008) 9–14. 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- [18].Huntzinger E, Izaurralde E, Gene silencing by microRNAs: contributions of translational repression and mRNA decay, Nat Rev Genet. 12 (2011) 99–110. 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- [19].Morgan CP, Bale TL, Sex differences in microRNA regulation of gene expression: no smoke, just miRs, Biol Sex Dif. 3 (2012) 22 10.1186/2042-6410-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guo L, Zhang Q, Ma X, Wang J, Liang T, miRNA and mRNA expression analysis reveals potential sex-biased miRNA expression, Sci Rep. 7 (2017) 39812 10.1038/srep39812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jin X-F, Wu N, Wang L, Li J, Circulating MicroRNAs: A Novel Class of Potential Biomarkers for Diagnosing and Prognosing Central Nervous System Diseases, Cell Mol Neurobiol. 33 (2013) 601–613. 10.1007/s10571-013-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Obrietan K, Hansen K, MicroRNA as therapeutic targets for treatment of depression, NDT. (2013) 1011 10.2147/NDT.S34811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Balakathiresan NS, Chandran R, Bhomia M, Jia M, Li H, Maheshwari RK, Serum and amygdala microRNA signatures of posttraumatic stress: Fear correlation and biomarker potential, Journal of Psychiatric Research. 57 (2014) 65–73. 10.1016/j.jpsychires.2014.05.020. [DOI] [PubMed] [Google Scholar]
- [24].Fan H, Sun X, Guo W, Zhong A, Niu W, Zhao L, Dai Y, Guo Z, Zhang L, Lu J, Differential expression of microRNA in peripheral blood mononuclear cells as specific biomarker for major depressive disorder patients, Journal of Psychiatric Research. 59 (2014) 45–52. 10.1016/j.jpsychires.2014.08.007. [DOI] [PubMed] [Google Scholar]
- [25].Lagarrigue E, 14_CH_8004_BA_INTERIEUR.qxd:DCNS#55, Translational Research. 16 (2014) 19. [Google Scholar]
- [26].Narahari A, Hussain M, Sreeram V, MicroRNAs as Biomarkers for Psychiatric Conditions:, (n.d.) 3. [PMC free article] [PubMed] [Google Scholar]
- [27].Maffioletti E, Cattaneo A, Rosso G, Maina G, Maj C, Gennarelli M, Tardito D, Bocchio-Chiavetto L, Peripheral whole blood microRNA alterations in major depression and bipolar disorder, Journal of Affective Disorders. 200 (2016) 250–258. 10.1016/j.jad.2016.04.021. [DOI] [PubMed] [Google Scholar]
- [28].Yuan H, Mischoulon D, Fava M, Otto MW, Circulating microRNAs as biomarkers for depression: Many candidates, few finalists, Journal of Affective Disorders. 233 (2018) 68–78. 10.1016/j.jad.2017.06.058. [DOI] [PubMed] [Google Scholar]
- [29].Fries GR, Zhang W, Benevenuto D, Quevedo J, MicroRNAs in Major Depressive Disorder, in: Guest PC (Ed.), Reviews on Biomarker Studies in Psychiatric and Neurodegenerative Disorders, Springer International Publishing, Cham, 2019: pp. 175–190. 10.1007/978-3-030-05542-4_9. [DOI] [Google Scholar]
- [30].Muiños-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipilä T, Maron E, Pettai K, Kananen L, Navinés R, Martín-Santos R, Gratacòs M, Metspalu A, Hovatta I, Estivill X, Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 Are Associated with Panic Disorder and Regulate Several Anxiety Candidate Genes and Related Pathways, Biological Psychiatry. 69 (2011) 526–533. 10.1016/j.biopsych.2010.10.010. [DOI] [PubMed] [Google Scholar]
- [31].Chen S, Sun X, Niu W, Kong L, He M, Fan H, Li W, Zhong A, Zhang L, Lu J, Correlation between the level of microRNA expression in peripheral blood mononuclear cells and symptomatology in patients with generalized anxiety disorder, Comprehensive Psychiatry. 69 (2016) 216–224. 10.1016/j.comppsych.2016.05.006. [DOI] [PubMed] [Google Scholar]
- [32].Pfau ML, Purushothaman I, Feng J, Golden SA, Aleyasin H, Lorsch ZS, Cates HM, Flanigan ME, Menard C, Heshmati M, Wang Z, Ma’ayan A, Shen L, Hodes GE, Russo SJ, Integrative Analysis of Sex-Specific microRNA Networks Following Stress in Mouse Nucleus Accumbens, Front. Mol. Neurosci 9 (2016). 10.3389/fnmol.2016.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Du K, Lu W, Sun Y, Feng J, Wang J-H, mRNA and miRNA profiles in the nucleus accumbens are related to fear memory and anxiety induced by physical or psychological stress, Journal of Psychiatric Research. 118 (2019) 44–65. 10.1016/j.jpsychires.2019.08.013. [DOI] [PubMed] [Google Scholar]
- [34].Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, Chen A, microRNA as Repressors of Stress-Induced Anxiety: The Case of Amygdalar miR-34, Journal of Neuroscience. 31 (2011) 14191–14203. 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Volk N, Pape JC, Engel M, Zannas AS, Cattane N, Cattaneo A, Binder EB, Chen A, Amygdalar MicroRNA-15a Is Essential for Coping with Chronic Stress, Cell Reports. 17 (2016) 1882–1891. 10.1016/j.celrep.2016.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun Y, Lu W, Du K, Wang J-H, microRNA and mRNA profiles in the amygdala are relevant to fear memory induced by physical or psychological stress, Journal of Neurophysiology. 122 (2019) 1002–1022. 10.1152/jn.00215.2019. [DOI] [PubMed] [Google Scholar]
- [37].Schmidt U, Herrmann L, Hagl K, Novak B, Huber C, Holsboer F, Wotjak CT, Buell DR, Therapeutic Action of Fluoxetine is Associated with a Reduction in Prefrontal Cortical miR-1971 Expression Levels in a Mouse Model of Posttraumatic Stress Disorder, Front. Psychiatry 4 (2013). 10.3389/fpsyt.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen RJ, Kelly G, Sengupta A, Heydendael W, Nicholas B, Beltrami S, Luz S, Peixoto L, Abel T, Bhatnagar S, MicroRNAs as biomarkers of resilience or vulnerability to stress, Neuroscience. 305 (2015) 36–48. 10.1016/j.neuroscience.2015.07.045. [DOI] [PubMed] [Google Scholar]
- [39].Dwivedi Y, Roy B, Lugli G, Rizavi H, Zhang H, Smalheiser NR, Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: relevance to depression pathophysiology, Transl Psychiatry. 5 (2015) e682–e682. 10.1038/tp.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mavrikaki M, Pantano L, Potter D, Rogers-Grazado MA, Anastasiadou E, Slack FJ, Amr SS, Ressler KJ, Daskalakis NP, Chartoff E, Sex-Dependent Changes in miRNA Expression in the Bed Nucleus of the Stria Terminalis Following Stress, Front. Mol. Neurosci 12 (2019) 236 10.3389/fnmol.2019.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Allen LS, Gorski RA, Sex difference in the bed nucleus of the stria terminalis of the human brain, J. Comp. Neurol 302 (1990) 697–706. 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- [42].Marcin MS, Nemeroff CB, The neurobiology of social anxiety disorder: the relevance of fear and anxiety, Acta Psychiatr Scand. 108 (2003) 51–64. 10.1034/j.1600-0447.108.s417.4.x. [DOI] [PubMed] [Google Scholar]
- [43].Walker DL, Toufexis DJ, Davis M, Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety, European Journal of Pharmacology. 463 (2003) 199–216. 10.1016/S0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- [44].Brühi AB, Delsignore A, Komossa K, Weidt S, Neuroimaging in social anxiety disorder—A meta-analytic review resulting in a new neurofunctional model, Neuroscience & Biobehavioral Reviews. 47 (2014) 260–280. 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- [45].Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, Trainor BC, Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice, Biological Psychiatry. 83 (2018) 203–213. 10.1016/j.biopsych.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fox AS, Shackman AJ, The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research, Neuroscience Letters. 693 (2019) 58–67. 10.1016/j.neulet.2017.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC, Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis, Frontiers in Behavioral Neuroscience. 7 (2014). 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mellios N, Huang H-S, Grigorenko A, Rogaev E, Akbarian S, A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex, Human Molecular Genetics. 17 (2008) 3030–3042. 10.1093/hmg/ddn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Im H-I, Hollander JA, Bali P, Kenny PJ, MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212, Nat Neurosci. 13 (2010) 1120–1127. 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Huang Y-WA, Ruiz CR, Eyler ECH, Lin K, Meffert MK, Dual Regulation of miRNA Biogenesis Generates Target Specificity in Neurotrophin-Induced Protein Synthesis, Cell. 148 (2012) 933–946. 10.1016/j.cell.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Leal G, Comprido D, Duarte CB, BDNF-induced local protein synthesis and synaptic plasticity, Neuropharmacology. 76 (2014) 639–656. 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- [52].Thornton JE, Gregory R.l., How does Lin28 let-7 control development and disease?, Trends in Cell Biology. 22 (2012) 474–482. 10.1016/j.tcb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ruiz CR, Shi J, Meffert MK, Transcript specificity in BDNF-regulated protein synthesis, Neuropharmacology. 76 (2014) 657–663. 10.1016/j.neuropharm.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Amen AM, Ruiz-Garzon CR, Shi J, Subramanian M, Pham DL, Meffert MK, A Rapid Induction Mechanism for Lin28a in Trophic Responses, Molecular Cell. 65 (2017) 490–503.e7. 10.1016/j.molcel.2016.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lei D, Shao Z, Zhou X, Yuan H, Synergistic neuroprotective effect of rasagiline and idebenone against retinal ischemia-reperfusion injury via the Lin28-let-7-Dicer pathway, Oncotarget. 9 (2018) 12137–12153. 10.18632/oncotarget.24343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pena JTG, Sohn-Lee C, Rouhanifard SH, Ludwig J, Hafner M, Mihailovic A, Lim C, Holoch D, Berninger P, Zavolan M, Tuschl T, miRNA in situ hybridization in formaldehyde and EDC-fixed tissues, Nat Methods. 6 (2009) 139–141. 10.1038/nmeth.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wan Y, Liu Y, Wang X, Wu J, Liu K, Zhou J, Liu L, Zhang C, Identification of Differential MicroRNAs in Cerebrospinal Fluid and Serum of Patients with Major Depressive Disorder, PLoS ONE. 10 (2015) e0121975 10.1371/journal.pone.0121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gururajan A, Naughton ME, Scott KA, O’Connor RM, Moloney G, Clarke G, Dowling J, Walsh A, Ismail F, Shorten G, Scott L, McLoughlin DM, Cryan JF, Dinan TG, MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c, Transl Psychiatry. 6 (2016) e862–e862. 10.1038/tp.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rinaldi A, Vincenti S, De Vito F, Bozzoni I, Oliverio A, Presutti C, Fragapane P, Mele A, Stress induces region specific alterations in microRNAs expression in mice, Behavioural Brain Research. 208 (2010) 265–269. 10.1016/j.bbr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- [60].Ribble DO, Salvioni M, Social Organization and Nest Co-Occupancy in Peromyscus californicus, a Monogamous Rodent, Behavioral Ecology and Sociobiology. 26 (1990) 9–15. [Google Scholar]
- [61].Davis ES, Marler CA, The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus, Hormones and Behavior. 44 (2003) 185–198. 10.1016/S0018-506X(03)00128-4. [DOI] [PubMed] [Google Scholar]
- [62].Silva AL, Fry WHD, Sweeney C, Trainor BC, Effects of photoperiod and experience on aggressive behavior in female California mice, Behavioural Brain Research. 208 (2010) 528–534. 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK, Sex Differences in Social Interaction Behavior Following Social Defeat Stress in the Monogamous California Mouse (Peromyscus californicus), PLoS ONE. 6 (2011) e17405 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Trainor BC, Martin LB, Greiwe KM, Kuhlman JR, Nelson RJ, Social and photoperiod effects on reproduction in five species of Peromyscus, General and Comparative Endocrinology. 148 (2006) 252–259. 10.1016/j.ygcen.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Trainor BC, Sima Finy M, Nelson RJ, Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice, Hormones and Behavior. 53 (2008) 192–199. 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC, Sex differences in effects of dopamine D1 receptors on social withdrawal, Neuropharmacology. 77 (2014) 208–216. 10.1016/j.neuropharm.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Magoc T, Salzberg SL, FLASH: fast length adjustment of short reads to improve genome assemblies, Bioinformatics. 27 (2011) 2957–2963. 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Friedländer MR, Chen W, Adamidi C, Maaskola J, Einspanier R, Knespel S, Rajewsky N, Discovering microRNAs from deep sequencing data using miRDeep, Nat Biotechnol. 26 (2008) 407–415. 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- [69].Friedländer MR, Mackowiak SD, Li N, Chen W, Rajewsky N, miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades, Nucleic Acids Research. 40 (2012) 37–52. 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kozomara A, Griffiths-Jones S, miRBase: integrating microRNA annotation and deep-sequencing data, Nucleic Acids Research. 39 (2011) D152–D157. 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sturm M, Hackenberg M, Langenberger D, Frishman D, TargetSpy: a supervised machine learning approach for microRNA target prediction, BMC Bioinformatics. 11 (2010) 292 10.1186/1471-2105-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sticht C, De La Torre C, Parveen A, Gretz N, miRWalk: An online resource for prediction of microRNA binding sites, PLoS ONE. 13 (2018) e0206239 10.1371/journal.pone.0206239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles G, Clark NR, Ma’ayan A, Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool, BMC Bioinformatics. 14 (2013) 128 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A, Enrichr: a comprehensive gene set enrichment analysis web server 2016 update, Nucleic Acids Res. 44 (2016) W90–W97. 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Plaisier SB, Taschereau R, Wong JA, Graeber TG, Rank–rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures, Nucleic Acids Research. 38 (2010) e169–e169. 10.1093/nar/gkq636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cahill KM, Huo Z, Tseng GC, Logan RW, Seney ML, Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach, Sci Rep. 8 (2018) 9588 10.1038/s41598-018-27903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chandrasekar V, Dreyer J-L, microRNAs miR-124, let-7d and miR-181a regulate Cocaine-induced Plasticity, Molecular and Cellular Neuroscience. 42 (2009) 350–362. 10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- [78].Thoenen H, Neurotrophins and Neuronal Plasticity, 270 (1995) 6. [DOI] [PubMed] [Google Scholar]
- [79].Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ, Anterograde transport of brain-derived neurotrophic factor and its role in the brain, Nature. 389 (1997) 856–860. 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- [80].Fanous S, Hammer RP, Nikulina EM, Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions, Neuroscience. 167 (2010) 598–607. 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dong H-W, Swanson LW, Projections from bed nuclei of the stria terminalis, anteromedial area: Cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance, J. Comp. Neurol 494 (2006) 142–178. 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Roush S, Slack FJ, The let-7 family of microRNAs, Trends in Cell Biology. 18 (2008) 505–516. 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [83].Lin R, Turecki G, Noncoding RNAs in Depression, in: Delgado-Morales R (Ed.), Neuroepigenomics in Aging and Disease, Springer International Publishing, Cham, 2017: pp. 197–210. 10.1007/978-3-319-53889-1_11. [DOI] [Google Scholar]
- [84].Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D, Changes in Brain MicroRNAs Contribute to Cholinergic Stress Reactions, J Mol Neurosci. 40 (2010) 47–55. 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bai M, Zhu X-Z, Zhang Y, Zhang S, Zhang L, Xue L, Zhong M, Zhang X, Anhedonia was associated with the dysregulation of hippocampal HTR4 and microRNA Let-7a in rats, Physiology & Behavior. 129 (2014) 135–141. 10.1016/j.physbeh.2014.02.035. [DOI] [PubMed] [Google Scholar]
- [86].Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH, Serotonin 5-HT 2C receptors regulate anxiety-like behavior, Genes, Brain and Behavior. 6 (2007) 491–496. 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- [87].Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, Kash TL, Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala, Nature. 537 (2016) 97–101. 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Pelrine E, Pasik SD, Bayat L, Goldschmiedt D, Bauer EP, 5-HT2C receptors in the BNST are necessary for the enhancement of fear learning by selective serotonin reuptake inhibitors, Neurobiology of Learning and Memory. 136 (2016) 189–195. 10.1016/j.nlm.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Saba R, Storchel PH, Aksoy-Aksel A, Kepura F, Lippi G, Plant TD, Schratt GM, Dopamine-Regulated MicroRNA MiR-181a Controls GluA2 Surface Expression in Hippocampal Neurons, Molecular and Cellular Biology. 32 (2012) 619–632. 10.1128/MCB.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Walker DL, Davis M, Double Dissociation between the Involvement of the Bed Nucleus of the Stria Terminalis and the Central Nucleus of the Amygdala in Startle Increases Produced by Conditioned versus Unconditioned Fear, J. Neurosci 17 (1997) 9375–9383. 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Song Y, Milon B, Ott S, Zhao X, Sadzewicz L, Shetty A, Boger ET, Tallon LJ, Morell RJ, Mahurkar A, Hertzano R, A comparative analysis of library prep approaches for sequencing low input translatome samples, BMC Genomics. 19 (2018) 696 10.1186/s12864-018-5066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sarantopoulou D, Tang SY, Ricciotti E, Lahens NF, Lekkas D, Schug J, Guo XS, Paschos GK, FitzGerald GA, Pack AI, Grant GR, Comparative evaluation of RNA-Seq library preparation methods for strand-specificity and low input, Sci Rep. 9 (2019) 13477 10.1038/s41598-019-49889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ, Sex differences in stress-induced social withdrawal: Independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding, Hormones and Behavior. 63 (2013) 543–550. 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, Hao R, Ramos-Maciel S, Rios RA, Jackson K, Flores-Ramirez FJ, Garcia-Carachure I, Pesavento PA, Iñiguez SD, Grinevich V, Trainor BC, Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance, Proc Natl Acad Sci USA. 117 (2020) 26406–26413. 10.1073/pnas.2011890117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wright EC, Johnson SA, Hao R, Kowalczyk AS, Greenberg GD, Ordoñes Sanchez E, Laman-Maharg A, Trainor BC, Rosenfeld CS, Exposure to extrinsic stressors, social defeat or bisphenol A, eliminates sex differences in DNA methyltransferase expression in the amygdala, J Neuroendocrinol. 29 (2017). 10.1111/jne.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kaur S, Kinkade JA, Green MT, Martin RE, Willemse TE, Bivens NJ, Schenk AK, Helferich WG, Trainor BC, Fass JN, M., Settles M, Mao J and Rosenfeld CR, in press Disruption of global hypothalamic microRNA (miR) profiles and associated behavioral changes in California mice (Peromyscus californicus) developmentally exposed to endocrine disrupting chemicals. Hormones and Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.