Introduction: the Challenge of Studying Nascent PCOS
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology (PCOM); it is also associated with metabolic comorbidities such as obesity and insulin resistance [1]. PCOS is a complex, multigenic disorder with important environmental determinants, but its etiology remains poorly understood [2–4].
The pathophysiology of symptomatic PCOS generally unfolds across puberty—the developmental stage during which increases in pulsatile GnRH release and gonadotropin secretion drive both ovarian sex steroid production and follicular development, with the eventual establishment of highly-complex feedback relationships that govern cyclic ovulation. However, the pubertal ontogeny of PCOS has been difficult to study because PCOS cannot be diagnosed prior to puberty, and diagnostic criteria appropriate for pubertal girls remain elusive [4, 5]. For example, pubertal hyperandrogenemia has not been clearly defined; hirsutism takes time to develop; oligomenorrhea in the 1–2 years after menarche is not by itself considered abnormal; and there are no validated criteria for PCOM in adolescent girls.
By the time a diagnosis of adolescent PCOS can be substantiated, the pathophysiology of PCOS appears to be largely, if not fully, entrenched. However, several groups appear to be at higher risk for developing adolescent PCOS—daughters of women with PCOS, girls with premature pubarche, and girls with obesity—and the study of such groups can offer important insights into the pubertal ontogeny of PCOS.
Etiology of PCOS: a Brief Overview
Some of the most carefully studied aspects of PCOS pathophysiology relate to abnormal ovarian function, insulin resistance and hyperinsulinemia, neuroendocrine dysfunction, and genetics. Additional factors include epigenetic modifications, adipose tissue dysfunction, inflammation, increased sympathetic nerve activity, environmental exposures, the microbiome, etc. [2–4]. The multitude of contributing factors likely play different relative roles in different subsets of patients with PCOS.
Abnormal Ovarian Steroidogenesis and Folliculogenesis
Adolescents and adults with PCOS exhibit abnormal patterns of sex steroid secretion (e.g., exaggerated 17-hydroxyprogesterone and androstenedione secretion) in response to acute GnRH agonist or human chorionic gonadotropin administration [3]. Abnormal steroidogenesis appears to be a stable property of ovarian theca cells in PCOS, partly reflecting increased expression of a splice variant of the DENND1A gene [3]. Similar abnormalities of adrenal sex steroid secretion are observed after exogenous ACTH administration, suggesting a more global dysregulation of steroidogenesis in PCOS [3]. Such abnormalities are exacerbated by factors such as hyperinsulinemia and disordered gonadotropin secretion [2].
PCOS is also characterized by abnormal follicular dynamics (enhanced follicular recruitment and later follicular arrest), which partly accounts for PCOM and likely involves, among other factors, abnormal gonadotropin action, intraovarian androgen excess, and hyperinsulinemia [2, 3, 6]. Moreover, anti-Müllerian hormone (AMH) concentrations—derived from granulosa cells in pre- and small antral ovarian follicles (2–9 mm)—are approximately 2- to 4-fold elevated in PCOS [7].
Insulin Resistance and Hyperinsulinemia
Women and adolescents with PCOS demonstrate metabolic insulin resistance that is exacerbated by, but partly independent of, obesity [8]. Insulin resistance may be further exacerbated during puberty, in part related to activation of the growth hormone axis [4]. The resulting compensatory hyperinsulinemia augments ovarian and adrenal androgen production, reduces hepatic SHBG production (increasing androgen bioavailability), and disrupts ovarian follicular dynamics and function [8]. Genetic studies implicate a number of metabolism-related genes including INSR (insulin receptor), INS-VNTR (insulin gene variable number of tandem repeats) and IRS1 (Insulin Receptor Substrate-1) [9]; and a recent Mendelian randomization study suggested that a genetic risk score for fasting insulin is associated with an increased risk of PCOS [10].
Reproductive Neuroendocrine Dysfunction
PCOS is associated with relative LH excess and FSH deficiency, both of which promote ovarian hyperandrogenism and impair follicular development [11]. Genetic studies support the relevance of genes related to gonadotropin secretion and action in PCOS, including FSHB (FSH subunit beta), FSHR (FSH receptor), and LHCGR (LH/choriogonadotropin receptor) [9]. Abnormal gonadotropin secretion in part relates to persistently high GnRH pulse frequency due to relative GnRH pulse generator resistance to negative feedback [11]. Although such negative feedback defects can be programmed by prenatal exposure to androgen excess, they appear to require ongoing androgen action in adulthood [11]. Recent studies also imply the potential role of AMH in the GnRH neuron dysfunction of PCOS [12, 13].
Insight from Animal Models with PCOS-Like Features
Investigators have developed a number of animal models with PCOS-like features [6], which permit invasive assessments and experimental manipulation that would be impractical, ethically unacceptable, or both, in girls and women. Although no animal model perfectly replicates PCOS, such animal models have substantially informed our understanding of PCOS pathophysiology.
Prenatally-androgenized (PNA) female rhesus macaques, sheep, and rodents demonstrate many PCOS-like features as adults [6]. For example, adult PNA female monkeys exhibit endogenous hyperandrogenemia, ovulatory dysfunction, polyfollicular ovaries, rapid GnRH pulsatility resistant to sex steroid negative feedback, abnormal gonadotropin secretion, increased adiposity, insulin resistance, impaired β-cell function, and dyslipidemia [6]. Yet complete or partial androgen receptor knockout prevents PCOS-like manifestations in PNA mice [14], suggesting the primacy of androgens in this regard. Recent findings in mice also suggest that the effects of prenatal androgenization can be transmitted through the germline in a transgenerational manner [15]. Although it remains unclear whether prenatal androgenization plays a role in PCOS, some human studies have supported this notion [6, 11].
Other animal models have been instructive as well. For example, peripubertal dihydrotestosterone treatment in female rodents produces many PCOS-like features such as ovulatory dysfunction, polyfollicular ovaries, increased adiposity, and insulin resistance [6]. Of interest, neuron-specific androgen receptor knockout prevented many of the untoward effects of postnatal dihydrotestosterone [16], suggesting the importance of central nervous system involvement in the pathophysiology of PCOS.
Studies in Girls at Risk for Developing PCOS
Within the ethical and practical limitations accompanying pediatric research, investigators have assessed the pubertal ontogeny of PCOS by characterizing the development of abnormalities in girls at high risk for adolescent PCOS: daughters of women with PCOS, girls with a history of premature pubarche, and girls with obesity.
Daughters of women with PCOS
Daughters of women with PCOS (PCOS-d) are expected to share PCOS susceptibility gene variants with their mothers; may be exposed to an intrauterine environment that enhances PCOS risk; and are likely to share postnatal environmental exposures with their mothers. A large Swedish registry study suggested that PCOS-d are approximately 5 times more likely to be diagnosed with PCOS as adults compared to control daughters; and a longitudinal cohort study suggested that Chilean PCOS-d are 10-fold more likely than control daughters to develop PCOS according to Rotterdam criteria [15].
Chilean daughters born to women with PCOS phenotype A (i.e., with hyperandrogenism, oligo-/amenorrhea, and PCOM) have been characterized in a number of studies (Table 1) [17–25]. As infants, these PCOS-d exhibited high serum AMH concentrations and exaggerated LH and estradiol responses to acute GnRH agonist stimulation [17, 22]. During childhood, Chilean PCOS-d exhibited elevated serum AMH, ovarian enlargement, and lower serum FSH concentrations [17, 19–21]. Although Chilean PCOS-d had no demonstrable hyperandrogenemia during childhood, a study of 1–3-year-old U.S. PCOS-d suggested increased 5α-reductase activity [26]. Moreover, Chilean PCOS-d exhibited exaggerated 17-hydroxyprogesterone and dehydroepiandrosterone (DHEA) responsiveness to exogenous ACTH during childhood (ages 4–8 years) and the peripubertal years (9–13 years), and approximately a third had exaggerated adrenarche [21]. Both AMH and ovarian volume appeared to be elevated throughout puberty in Chilean PCOS-d [20, 24]. However, many of the classic reproductive abnormalities of PCOS—elevated testosterone, free androgen index, basal LH, LH/FSH ratio; lower SHBG; and exaggerated 17-hydroxyprogesterone and testosterone responses to acute GnRH agonist challenge—manifested only toward the end of puberty (e.g., Tanner stage 4) [20, 24, 25].
Table 1.
Selected findings in Chilean daughters of women with PCOS
| Infancy (age 2–3 months) | Mid- to late childhood; prepubertal | Early puberty (Tanner 2–3) | Late puberty (Tanner 4–5 and/or postmen archeal) | Peripubertal (age 8–16 years, Tanner 1–5) |
|---|---|---|---|---|
| References 17, 22 | References 17, 18, 20, 21, 23, 24 | References 20, 23, 24 | References 20, 23–25 | References 18, 19, 21 |
| − | − | − | + | + |
| − | − | − | + | +/− |
| − | − | + | + | |
| − | − | − | − | − |
| − | − | − | (−) | − |
| − | + | |||
| − | − | + | ||
| − | − | − | +/− | |
| − | + | |||
| − | − | − | +/− | |
| + | ||||
| + | + | |||
| + | + | |||
| − | − | − | (−) | − |
| − | (−) | − | − | − |
| − | − | (−) | ||
| + | − | − | + | |
| + | + | + | + | + |
| + | + | + | + | |
| − | − | − | − | − |
| − | − | − | − | − |
| − | − | − | − | − |
| − | − | (−) | − | |
| − | − | − | − | |
| + | + | + | + | |
| − | − | +/− | ||
| − | − | +/− | + | |
| + | − | − | − | |
| − | − | − | − |
Key: “+” = available data suggests presence; “+/−” = data mixed; “(−)” = most but not all data suggests against presence; “−” = available data suggests against presence; blank = data not reported in these studies.
Studies in peripubertal PCOS-d from the U.S. have supported some, but not all of the above findings (Table 2) [26–30]. In two U.S. studies of pre- and early pubertal PCOS-d, AMH levels were not significantly elevated compared to controls, while estimates of free testosterone were increased [28, 30]. Another study suggested no differences in urinary steroids, urinary gonadotropins, or ovarian volume in peripubertal PCOS-d [29].
Table 2.
Selected findings in North American daughters (PCOS-d) or first-degree relatives (PCOS-fdr) of women with PCOS
| Young childhood (age 1–3 years) | Pre- and early pubertal (age 8–12 years; Tanner 1–3; premenarcheal) | Mid-childhood (prepubertal) | Early pubertal (Tanner 2–3) | Late pubertal (Tanner 4–5) | Peripubertal (age 8–14 years) |
|---|---|---|---|---|---|
| PCOS-d from Illinois, U.S. | PCOS-d and PCOS-fdr from Illinois and Pennsylvania, U.S. | PCOS-d from Pennsylvania, U.S. | PCOS-fdr from Quebec, Canada | ||
| Reference 26 | References 28 and 30 | Reference 29 | Reference 27 | ||
| +/− | − | − | − | − | |
| +/− | + | ||||
| + | − | ||||
| − | − | − | − | − | |
| − | − | − | − | ||
| − | − | − | − | ||
| + | |||||
| − | |||||
| − | − | + | |||
| − | − | − | − | ||
| − | − | − | − | ||
| − | − | − | |||
| − | |||||
| − | − | − | |||
| + | +/− | − | − | − | + |
| − | − | +/− | − | − | |
| − | + | ||||
| − | − | ||||
| − | − | − | − | + | |
| + | |||||
| + | |||||
| − | + | ||||
| + | + | ||||
| − | − | ||||
| − | |||||
| + | |||||
Key: “+” = available data suggests presence; “+/−” = data mixed; “−” = available data suggests against presence; blank = data not reported in these studies.
PCOS-d also exhibit early metabolic abnormalities (Tables 1 and 2). For example, Chilean PCOS-d exhibit exaggerated serum insulin responses to an oral glucose challenge during childhood and puberty, despite no clear anthropometric abnormalities (e.g., body mass index [BMI] z-score, waist-to-hip ratio) [17–21, 23–25]. Similarly, female 8–14-year-old peripubertal first degree relatives of U.S. women with PCOS (PCOS-fdr) exhibited insulin resistance, exaggerated insulin responses to an oral glucose load, and impaired β-cell function—differences that remained after adjusting for differences in BMI z-score [27].
Girls with premature and/or exaggerated adrenarche
Adrenarche represents the developmental rise in adrenal androgen production, heralded clinically by the development of pubic hair (pubarche), axillary hair, and apocrine odor [31]. Premature pubarche—occurring earlier than age 8 years in girls—is typically related to premature/exaggerated adrenarche and is a common cause of androgen excess in prepubertal children [31, 32].
Premature and/or exaggerated adrenarche may also represent a harbinger of PCOS. Ibanez and colleagues initially observed that approximately half of Catalan (northeastern Spanish) girls with premature pubarche developed post-pubertal oligomenorrhea, hirsutism, hyperandrogenemia, and functional ovarian hyperandrogenism in response to acute GnRH agonist testing [33]. The emergence of PCOS-like abnormalities across puberty was assessed in a number of subsequent studies (Table 3) [34–39]. Prepubertal Catalan girls with a history of premature pubarche exhibited elevated free androgen index, DHEA sulfate (DHEAS), and insulin-like growth factor-1 (IGF-1); hyperinsulinemia after an oral glucose load; and increased total and central adiposity despite normal BMI. These latter two abnormalities were observed throughout puberty. While elevations in total testosterone, free androgen index, and androstenedione were not observed in early puberty, hyperandrogenemia was demonstrable in later puberty. Similarly, while such girls demonstrated variably elevated 17-hydroxypregnenolone and DHEA responses to acute GnRH agonist stimulation throughout puberty, the more typical findings of functional ovarian hyperandrogenism (e.g., exaggerated 17-hydroxyprogesterone and androstenedione responses to acute GnRH agonism) were not apparent until late puberty [34]. Abnormal ovulatory dysfunction became evident after 3-years postmenarche [40].
Table 3.
Selected findings in Catalan girls with a history of premature pubarche
| Mid- to late childhood; prepubertal | Early puberty (Tanner 2; premenarcheal) | Early/mid-puberty (Tanner 3; premenarcheal) | Late puberty (Tanner 4–5 and postmenarcheal) |
|---|---|---|---|
| References 35–37, 39 | References 34–37, 39 | References 34–37, 39 | References 34–39 |
| + | − | +/− | + |
| +/− | +/− | +/− | + |
| + | − | + | + |
| − | − | + | |
| − | − | +/− | |
| + | + | − | (+) |
| + | − | + | |
| − | + | + | |
| + | + | +/− | |
| + | − | +/− | |
| − | − | +/− | |
| − | − | +/− | |
| − | − | + | |
| − | − | +/− | |
| − | − | + | |
| − | − | + | |
| − | |||
| + | |||
| + | |||
| − | − | +/− | |
| − | − | − | |
| − | − | − | |
| − | (−) | (−) | − |
| + | + | + | + |
| + | + | + | + |
| + | + | + | + |
| + | + | − | + |
| + | + | + | + |
| + | − | − | + |
| + | + | + | + |
| − | − | +/− | +/− |
| + | − | − | − |
| + | − | − | + |
| +/− | +/− | +/− | + |
Key: “+” = available data suggests presence; “(+)” = most but not all data suggests presence; “+/−” = data mixed; “(−)” = most but not all data suggests against presence; “−” = available data suggests against presence; blank = data not reported in these studies.
A quarter to a third of women with PCOS have evidence for adrenal hyperandrogenemia [41], and the potent androgen 11-ketotestosterone predominates among circulating androgens in both girls with premature adrenarche and in women with PCOS [42, 43]. Accordingly, the link between premature/exaggerated adrenarche and PCOS may partly reflect general abnormalities of androgen steroidogenesis, manifesting as adrenal hyperandrogenemia at adrenarche and ovarian hyperandrogenemia at puberty. Premature/exaggerated adrenarche and adolescent PCOS are also linked to increased visceral fat, hyperinsulinemia, and increased free IGF-1 concentrations, with the latter two augmenting adrenal and ovarian theca cell androgen production [3, 39, 44–48]. In Catalan girls with premature pubarche, a history of small for gestational age (SGA) was associated with a more severe phenotype in late puberty [37]. In these girls, peripubertal treatment with metformin reduced total and visceral adiposity, IGF-1 levels, and reduced the risk of hyperandrogenism, oligomenorrhea, and PCOS [49–51].
Peripubertal girls with obesity
Obesity is associated with menstrual dysfunction and higher free testosterone concentrations in women with and without PCOS, and weight loss can ameliorate the manifestations of PCOS in adolescents and adults with obesity [52]. Additionally, PCOS has been associated with a high prevalence of obesity [53], although referral bias partly accounts for this finding [54]. In a large population-based study of 15–19-year-old adolescent girls in the U.S., the prevalence of PCOS was estimated to be 3.0-, 6.7-, and 14.7-fold elevated in girls with overweight, moderate obesity, and more extreme obesity, respectively [55]. Furthermore, in a large meta-analysis of PCOS cases and controls of European descent, linkage disequilibrium-score regression suggested that both childhood obesity and BMI are genetically correlated with an increased risk of PCOS [10]. Mendelian randomization analyses also suggest that genetic risk scores for BMI are associated with PCOS [10, 56, 57].
Free testosterone levels are approximately 2- to 4-fold higher in peripubertal girls with obesity compared to non-obese controls [30, 58–62]. One study suggested that total testosterone was 4-fold elevated in prepubertal girls with obesity [58], while another suggested that free testosterone was over 8-fold elevated in prepubertal girls with obesity, with absolute levels similar to those in Tanner 5 girls without obesity [59]. Although some studies imply that higher free testosterone is largely attributable to reduced SHBG in these girls [30, 62], others demonstrated elevated total testosterone as well [58, 59, 61], suggesting increased production. Some [58, 61, 63] but not all [30, 62] studies suggest that peripubertal obesity is also associated with adrenal hyperandrogenemia.
Obesity presumably contributes to the pubertal ontogeny of PCOS partly via insulin resistance and compensatory hyperinsulinemia [62]. Some investigators have hypothesized that SGA (intrauterine growth retardation) with postnatal catch-up growth and/or excessive postnatal weight gain in non-SGA children contributes to hepatovisceral fat excess, which increases risk for both premature pubarche and PCOS via insulin resistance, hyperinsulinemia, IGF-1 excess, and hypoadiponectinemia [64, 65]. Moreover, excess adiposity may be associated with altered peripheral steroid metabolism that can enhance hyperandrogenemia (e.g., increased 17β-hydroxysteroid dehydrogenase type 5 activity, increased 5α-reductase activity, altered cortisol metabolism) [52].
Interestingly, available data suggest that early pubertal girls with obesity exhibit reduced LH release without the expected overnight changes in LH pulse frequency and amplitude [66–69], while late pubertal girls with obesity exhibit elevated day and night LH pulse frequency without the expected overnight decrease [66]. The latter finding appears to be related to hyperandrogenemia specifically [69]. Also of interest, LH concentration appeared to be a better predictor of free testosterone than insulin concentration in peripubertal girls with obesity [60, 70].
Recent studies indicate that serum AMH concentration, as a correlate of ovarian antral follicle number, is an important marker of PCOS risk in adolescent girls with obesity. In one study, AMH was 1.9-fold higher in adolescent girls with obesity and PCOS compared to adolescent girls with obesity alone [71]. In another study, girls with obesity born to non-PCOS mothers had similar free testosterone, androstenedione, and DHEAS levels compared to PCOS-d, but PCOS-d—presumed to be at higher risk for PCOS—had 2.7-fold higher AMH [30].
Hypothetical Model for the Pubertal Ontogeny of PCOS
A hypothetical model for the pubertal ontogeny of PCOS is represented in Figure 1. The model involves a genetic predisposition and possible intrauterine programming that promotes childhood hyperinsulinism, exaggerated ovarian and adrenal androgen steroidogenesis in response to stimulation, and post-pubertal neuroendocrine dysfunction. At adrenarche, ACTH stimulates adrenals primed to secrete excess androgen; at neuroendocrine puberty, gonadotropins stimulate ovaries primed to secrete excess androgens; and both of these phenomena are enhanced by hyperinsulinemia and elevated IGF-1 levels. Moreover, entry into neuroendocrine puberty in a hyperandrogenemic milieu impairs negative feedback at the GnRH pulse generator. This, along with elevated AMH, promotes rapid GnRH pulse frequency, increasing LH and limiting FSH release. These neuroendocrine defects further enhance hyperandrogenemia and impair ovulatory function, supporting a progression to full-blown PCOS. Although not represented in the necessarily-simplistic illustration (Figure 1), numerous other factors contribute to various nodes and circuits underlying PCOS pathophysiology.
Figure 1.
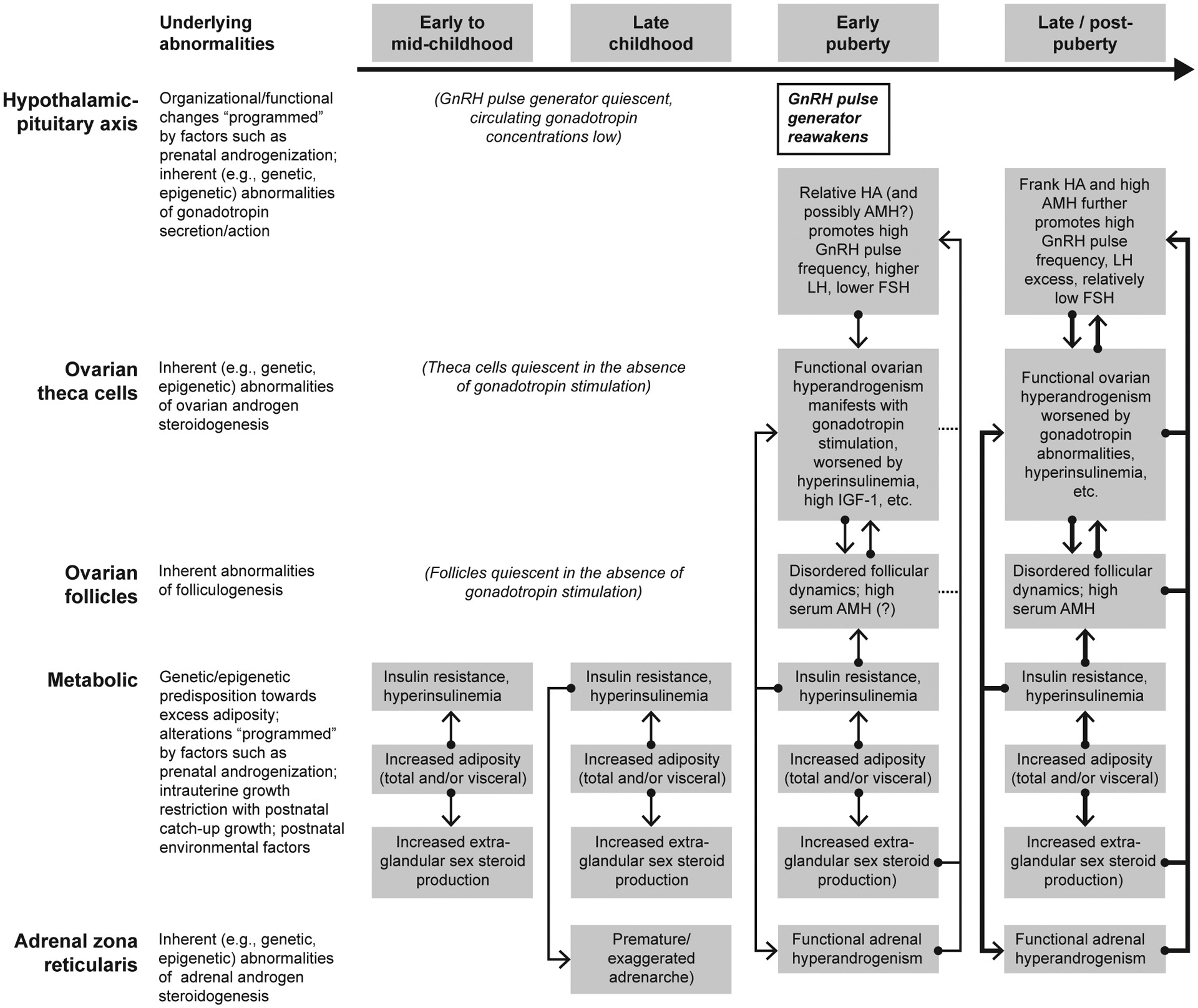
Hypothetical model for the pubertal ontogeny of PCOS. HA = hyperandrogenemia.
Concluding Remarks
It is not currently possible to diagnose pre-symptomatic, or even early symptomatic, PCOS in peripubertal girls. However, the study of peripubertal girls at high risk for PCOS has provided important insights into the developmental pathophysiology of PCOS. In addition to enhancing our fundamental understanding of PCOS, such research may suggest novel preventive strategies. These considerations underscore the importance of continued research into the pubertal ontogeny of PCOS.
Key Points:
The initial manifestations of PCOS often arise during or shortly after puberty, presumably related to the pubertal increase in gonadotropins and, in turn, ovarian androgen production.
The pubertal ontogeny of PCOS is difficult to study in humans because the pathophysiology is typically well entrenched before the diagnosis can be substantiated.
Several groups appear to be at higher risk for developing adolescent PCOS—daughters of women with PCOS, girls with premature pubarche, and girls with obesity—and the study of these groups can offer insight into the pubertal ontogeny of PCOS.
Available data supports the hypothesis that the pubertal etiology of PCOS involves various combinations of genetic predisposition, intrauterine programming, hyperinsulinism, and other abnormalities, that provoke reproductive symptoms (e.g., hyperandrogenism, ovulatory dysfunction) in response to the pubertal increase in gonadotropin secretion.
Synopsis:
The pathophysiology of symptomatic PCOS often unfolds across puberty, but the ontogeny of PCOS is difficult to study because, in general, its pathophysiology is well entrenched before the diagnosis can be confirmed. However, the study of high-risk groups—daughters of women with PCOS, girls with premature pubarche, and girls with obesity—can offer insight in this regard. Available data supports the hypothesis that the pubertal etiology of PCOS involves various combinations of genetic predisposition, intrauterine programming, hyperinsulinism, and numerous other abnormalities, that provoke reproductive symptoms (e.g., hyperandrogenism, ovulatory dysfunction) in response to the pubertal increase in gonadotropin secretion.
Clinics Care Points.
Symptoms and signs of PCOS may initially manifest during puberty, but it is not currently possible to diagnose pre-symptomatic, or even early symptomatic, PCOS in peripubertal girls.
Certain populations of girls are at higher risk for adolescent PCOS and should be monitored by symptoms: daughters of women with PCOS, girls with premature adrenarche, and girls with obesity.
Definitive recommendations for prevention or early treatment in young girls at risk for PCOS are not available due to poorly understood early etiologies.
Given that insulin resistance has been associated with the development of PCOS, healthy lifestyle (e.g., diet, exercise) and maintenance of healthy weight are presumably important goals for adolescents at higher risk for PCOS; however, such approaches would not address all possible etiological mechanisms.
For peripubertal adolescents with potential symptoms or signs of nascent PCOS, but who do not clearly meet diagnostic criteria, continued follow-up is prudent so that the diagnosis is not unnecessarily delayed.
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through R01 HD102060 (CBS, CRM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
References
- 1.McCartney CR and Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med 2016; 375(1): 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, and Legro RS. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev 2015; 36(5): 487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfield RL and Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev 2016; 37(5): 467–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibanez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, Dabadghao P, Darendeliler F, Elbarbary NS, Gambineri A, Garcia Rudaz C, Hoeger KM, Lopez-Bermejo A, Ong K, Pena AS, Reinehr T, Santoro N, Tena-Sempere M, Tao R, Yildiz BO, Alkhayyat H, Deeb A, Joel D, Horikawa R, de Zegher F, and Lee PA. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm Res Paediatr 2017; 88(6): 371–395. [DOI] [PubMed] [Google Scholar]
- 5.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, and International PN. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018; 110(3): 364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, Dumesic DA, and Abbott DH. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr Rev 2020; 41(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumont A, Robin G, and Dewailly D. Anti-mullerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome. Curr Opin Endocrinol Diabetes Obes 2018; 25(6): 377–384. [DOI] [PubMed] [Google Scholar]
- 8.Diamanti-Kandarakis E and Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012; 33(6): 981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiam D, Moreno-Asso A, Teede HJ, Laven JSE, Stepto NK, Moran LJ, and Gibson-Helm M. The Genetics of Polycystic Ovary Syndrome: An Overview of Candidate Gene Systematic Reviews and Genome-Wide Association Studies. J Clin Med 2019; 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, Kraft P, Lin N, Huang H, Broer L, Magi R, Saxena R, Laisk T, Urbanek M, Hayes MG, Thorleifsson G, Fernandez-Tajes J, Mahajan A, Mullin BH, Stuckey BGA, Spector TD, Wilson SG, Goodarzi MO, Davis L, Obermayer-Pietsch B, Uitterlinden AG, Anttila V, Neale BM, Jarvelin MR, Fauser B, Kowalska I, Visser JA, Andersen M, Ong K, Stener-Victorin E, Ehrmann D, Legro RS, Salumets A, McCarthy MI, Morin-Papunen L, Thorsteinsdottir U, Stefansson K, andMe Research T, Styrkarsdottir U, Perry JRB, Dunaif A, Laven J, Franks S, Lindgren CM, and Welt CK. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet 2018; 14(12): e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCartney CR and Campbell RE. Central Nervous System Control in PCOS. Current Opinion in Endocrine and Metabolic Research 2020; 12: 78–84 [DOI: 10.1016/j.coemr.2020.04.005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D, Pigny P, Prescott M, Campbell R, Herbison AE, Prevot V, and Giacobini P. Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun 2016; 7: 10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundstrom-Poromaa I, Piltonen TT, Dal Bello F, Medana C, Prevot V, Clasadonte J, and Giacobini P. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med 2018; 24(6): 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldwell AS, Eid S, Kay CR, Jimenez M, McMahon AC, Desai R, Allan CM, Smith JT, Handelsman DJ, and Walters KA. Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology 2015; 156(4): 1441–52. [DOI] [PubMed] [Google Scholar]
- 15.Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladron de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, and Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med 2019; 25(12): 1894–1904. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, and Walters KA. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci U S A 2017; 114(16): E3334–E3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sir-Petermann T, Codner E, Maliqueo M, Echiburu B, Hitschfeld C, Crisosto N, Perez-Bravo F, Recabarren SE, and Cassorla F. Increased anti-Mullerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006; 91(8): 3105–9. [DOI] [PubMed] [Google Scholar]
- 18.Crisosto N, Codner E, Maliqueo M, Echiburu B, Sanchez F, Cassorla F, and Sir-Petermann T. Anti-Mullerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007; 92(7): 2739–43. [DOI] [PubMed] [Google Scholar]
- 19.Sir-Petermann T, Maliqueo M, Codner E, Echiburu B, Crisosto N, Perez V, Perez-Bravo F, and Cassorla F. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007; 92(12): 4637–42. [DOI] [PubMed] [Google Scholar]
- 20.Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron de Guevara A, Preisler J, Crisosto N, Sanchez F, Cassorla F, and Bhasin S. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009; 94(6): 1923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maliqueo M, Sir-Petermann T, Perez V, Echiburu B, de Guevara AL, Galvez C, Crisosto N, and Azziz R. Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009; 94(9): 3282–8. [DOI] [PubMed] [Google Scholar]
- 22.Crisosto N, Echiburu B, Maliqueo M, Perez V, Ladron de Guevara A, Preisler J, Sanchez F, and Sir-Petermann T. Improvement of hyperandrogenism and hyperinsulinemia during pregnancy in women with polycystic ovary syndrome: possible effect in the ovarian follicular mass of their daughters. Fertil Steril 2012; 97(1): 218–24. [DOI] [PubMed] [Google Scholar]
- 23.Maliqueo M, Galgani JE, Perez-Bravo F, Echiburu B, de Guevara AL, Crisosto N, and Sir-Petermann T. Relationship of serum adipocyte-derived proteins with insulin sensitivity and reproductive features in pre-pubertal and pubertal daughters of polycystic ovary syndrome women. Eur J Obstet Gynecol Reprod Biol 2012; 161(1): 56–61. [DOI] [PubMed] [Google Scholar]
- 24.Sir-Petermann T, Ladron de Guevara A, Codner E, Preisler J, Crisosto N, Echiburu B, Maliqueo M, Sanchez F, Perez-Bravo F, and Cassorla F. Relationship between anti-Mullerian hormone (AMH) and insulin levels during different tanner stages in daughters of women with polycystic ovary syndrome. Reprod Sci 2012; 19(4): 383–90. [DOI] [PubMed] [Google Scholar]
- 25.Crisosto N, Ladron de Guevara A, Echiburu B, Maliqueo M, Cavada G, Codner E, Paez F, and Sir-Petermann T. Higher luteinizing hormone levels associated with antimullerian hormone in postmenarchal daughters of women with polycystic ovary syndrome. Fertil Steril 2019; 111(2): 381–388. [DOI] [PubMed] [Google Scholar]
- 26.Torchen LC, Idkowiak J, Fogel NR, O’Neil DM, Shackleton CH, Arlt W, and Dunaif A. Evidence for Increased 5alpha-Reductase Activity During Early Childhood in Daughters of Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2016; 101(5): 2069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trottier A, Battista MC, Geller DH, Moreau B, Carpentier AC, Simoneau-Roy J, and Baillargeon JP. Adipose tissue insulin resistance in peripubertal girls with first-degree family history of polycystic ovary syndrome. Fertil Steril 2012; 98(6): 1627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torchen LC, Fogel NR, Brickman WJ, Paparodis R, and Dunaif A. Persistent apparent pancreatic beta-cell defects in premenarchal PCOS relatives. J Clin Endocrinol Metab 2014; 99(10): 3855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legro RS, Kunselman AR, Stetter CM, Gnatuk CL, Estes SJ, Brindle E, Vesper HW, Botelho JC, Lee PA, and Dodson WC. Normal Pubertal Development in Daughters of Women With PCOS: A Controlled Study. J Clin Endocrinol Metab 2017; 102(1): 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torchen LC, Legro RS, and Dunaif A. Distinctive Reproductive Phenotypes in Peripubertal Girls at Risk for Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2019; 104(8): 3355–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idkowiak J, Lavery GG, Dhir V, Barrett TG, Stewart PM, Krone N, and Arlt W. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol 2011; 165(2): 189–207. [DOI] [PubMed] [Google Scholar]
- 32.Idkowiak J, Elhassan YS, Mannion P, Smith K, Webster R, Saraff V, Barrett TG, Shaw NJ, Krone N, Dias RP, Kershaw M, Kirk JM, Hogler W, Krone RE, O’Reilly MW, and Arlt W. Causes, patterns and severity of androgen excess in 487 consecutively recruited pre- and post-pubertal children. Eur J Endocrinol 2019; 180(3): 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibanez L, Potau N, Virdis R, Zampolli M, Terzi C, Gussinye M, Carrascosa A, and Vicens-Calvet E. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab 1993; 76(6): 1599–603. [DOI] [PubMed] [Google Scholar]
- 34.Ibanez L, Potau N, Zampolli M, Street ME, and Carrascosa A. Girls diagnosed with premature pubarche show an exaggerated ovarian androgen synthesis from the early stages of puberty: evidence from gonadotropin-releasing hormone agonist testing. Fertil Steril 1997; 67(5): 849–55. [DOI] [PubMed] [Google Scholar]
- 35.Ibanez L, Potau N, Zampolli M, Rique S, Saenger P, and Carrascosa A. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J Clin Endocrinol Metab 1997; 82(7): 2283–8. [DOI] [PubMed] [Google Scholar]
- 36.Ibanez L, Potau N, Chacon P, Pascual C, and Carrascosa A. Hyperinsulinaemia, dyslipaemia and cardiovascular risk in girls with a history of premature pubarche. Diabetologia 1998; 41(9): 1057–63. [DOI] [PubMed] [Google Scholar]
- 37.Ibanez L, Potau N, Francois I, and de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab 1998; 83(10): 3558–62. [DOI] [PubMed] [Google Scholar]
- 38.Ibanez L, Potau N, Marcos MV, and De Zegher F. Adrenal hyperandrogenism in adolescent girls with a history of low birthweight and precocious pubarche. Clin Endocrinol (Oxf) 2000; 53(4): 523–7. [DOI] [PubMed] [Google Scholar]
- 39.Ibanez L, Ong K, de Zegher F, Marcos MV, del Rio L, and Dunger DB. Fat distribution in non-obese girls with and without precocious pubarche: central adiposity related to insulinaemia and androgenaemia from prepuberty to postmenarche. Clin Endocrinol (Oxf) 2003; 58(3): 372–9. [DOI] [PubMed] [Google Scholar]
- 40.Ibanez L, de Zegher F, and Potau N. Anovulation after precocious pubarche: early markers and time course in adolescence. J Clin Endocrinol Metab 1999; 84(8): 2691–5. [DOI] [PubMed] [Google Scholar]
- 41.Rosenfield RL, Mortensen M, Wroblewski K, Littlejohn E, and Ehrmann DA. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Hum Reprod 2011; 26(11): 3138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, and Arlt W. 11-Oxygenated C19 Steroids Are the Predominant Androgens in Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2017; 102(3): 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rege J, Turcu AF, Kasa-Vubu JZ, Lerario AM, Auchus GC, Auchus RJ, Smith JM, White PC, and Rainey WE. 11-Ketotestosterone Is the Dominant Circulating Bioactive Androgen During Normal and Premature Adrenarche. J Clin Endocrinol Metab 2018; 103(12): 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuguin P, Linder B, Rosenfeld RG, Saenger P, and DiMartino-Nardi J. The roles of insulin sensitivity, insulin-like growth factor I (IGF-I), and IGF-binding protein-1 and −3 in the hyperandrogenism of African-American and Caribbean Hispanic girls with premature adrenarche. J Clin Endocrinol Metab 1999; 84(6): 2037–42. [DOI] [PubMed] [Google Scholar]
- 45.Silfen ME, Manibo AM, Ferin M, McMahon DJ, Levine LS, and Oberfield SE. Elevated free IGF-I levels in prepubertal Hispanic girls with premature adrenarche: relationship with hyperandrogenism and insulin sensitivity. J Clin Endocrinol Metab 2002; 87(1): 398–403. [DOI] [PubMed] [Google Scholar]
- 46.Silfen ME, Denburg MR, Manibo AM, Lobo RA, Jaffe R, Ferin M, Levine LS, and Oberfield SE. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab 2003; 88(10): 4682–8. [DOI] [PubMed] [Google Scholar]
- 47.Guven A, Cinaz P, and Bideci A. Is premature adrenarche a risk factor for atherogenesis? Pediatr Int 2005; 47(1): 20–5. [DOI] [PubMed] [Google Scholar]
- 48.Utriainen P, Jaaskelainen J, Romppanen J, and Voutilainen R. Childhood metabolic syndrome and its components in premature adrenarche. J Clin Endocrinol Metab 2007; 92(11): 4282–5. [DOI] [PubMed] [Google Scholar]
- 49.Ibanez L, Ong K, Valls C, Marcos MV, Dunger DB, and de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab 2006; 91(8): 2888–91. [DOI] [PubMed] [Google Scholar]
- 50.Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV, and de Zegher F. Metformin treatment for four years to reduce total and visceral fat in low birth weight girls with precocious pubarche. J Clin Endocrinol Metab 2008; 93(5): 1841–5. [DOI] [PubMed] [Google Scholar]
- 51.Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV, and de Zegher F. Early metformin therapy (age 8–12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J Clin Endocrinol Metab 2011; 96(8): E1262–7. [DOI] [PubMed] [Google Scholar]
- 52.Anderson AD, Solorzano CM, and McCartney CR. Childhood Obesity and Its Impact on the Development of Adolescent PCOS. Semin Reprod Med 2014; 32(3): 202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim SS, Davies MJ, Norman RJ, and Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012; 18(6): 618–37. [DOI] [PubMed] [Google Scholar]
- 54.Lizneva D, Kirubakaran R, Mykhalchenko K, Suturina L, Chernukha G, Diamond MP, and Azziz R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: systematic review and meta-analysis. Fertil Steril 2016. [DOI] [PubMed] [Google Scholar]
- 55.Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, and Koebnick C. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril 2013; 100(2): 470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, Bjonnes A, Broer L, Dunger DB, Halldorsson BV, Lawlor DA, Laval G, Mathieson I, McCardle WL, Louwers Y, Meun C, Ring S, Scott RA, Sulem P, Uitterlinden AG, Wareham NJ, Thorsteinsdottir U, Welt C, Stefansson K, Laven JSE, Ong KK, and Perry JRB. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun 2015; 6: 8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brower MA, Hai Y, Jones MR, Guo X, Chen YI, Rotter JI, Krauss RM, Legro RS, Azziz R, and Goodarzi MO. Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum Reprod 2019; 34(1): 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reinehr T, de Sousa G, Roth CL, and Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab 2005; 90(10): 5588–95. [DOI] [PubMed] [Google Scholar]
- 59.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, and Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007; 92(2): 430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knudsen KL, Blank SK, Burt Solorzano C, Patrie JT, Chang RJ, Caprio S, Marshall JC, and McCartney CR. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity (Silver Spring) 2010; 18(11): 2118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang MJ, Yang S, and Hwang IT. The impact of obesity on hyperandrogenemia in Korean girls. Ann Pediatr Endocrinol Metab 2016; 21(4): 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nokoff N, Thurston J, Hilkin A, Pyle L, Zeitler PS, Nadeau KJ, Santoro N, and Kelsey MM. Sex Differences in Effects of Obesity on Reproductive Hormones and Glucose Metabolism in Early Puberty. J Clin Endocrinol Metab 2019; 104(10): 4390–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burt Solorzano CM, Helm KD, Patrie JT, Shayya RF, Cook-Andersen HL, Chang RJ, McCartney CR, and Marshall JC. Increased Adrenal Androgens in Overweight Peripubertal Girls. J Endocr Soc 2017; 1(5): 538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Zegher F, Reinehr T, Malpique R, Darendeliler F, Lopez-Bermejo A, and Ibanez L. Reduced Prenatal Weight Gain and/or Augmented Postnatal Weight Gain Precedes Polycystic Ovary Syndrome in Adolescent Girls. Obesity (Silver Spring) 2017; 25(9): 1486–1489. [DOI] [PubMed] [Google Scholar]
- 65.de Zegher F, Lopez-Bermejo A, and Ibanez L. Central Obesity, Faster Maturation, and ‘PCOS’ in Girls. Trends Endocrinol Metab 2018; 29(12): 815–818. [DOI] [PubMed] [Google Scholar]
- 66.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, and Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab 2009; 94(1): 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bordini B, Littlejohn E, and Rosenfield RL. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab 2009; 94(4): 1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenfield RL, Bordini B, and Yu C. Comparison of Detection of Normal Puberty in Girls by a Hormonal Sleep Test and a Gonadotropin-Releasing Hormone Agonist Test. J Clin Endocrinol Metab 2013; 98(4): 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collins JS, Beller JP, Burt Solorzano C, Patrie JT, Chang RJ, Marshall JC, and McCartney CR. Blunted day-night changes in luteinizing hormone pulse frequency in girls with obesity: the potential role of hyperandrogenemia. J Clin Endocrinol Metab 2014; 99(8): 2887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burt Solorzano CM, Knudsen KL, Anderson AD, Hutchens EG, Collins JS, Patrie JT, Marshall JC, and McCartney CR. Insulin Resistance, Hyperinsulinemia, and LH: Relative Roles in Peripubertal Obesity-Associated Hyperandrogenemia. J Clin Endocrinol Metab 2018; 103(7): 2571–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim JY, Tfayli H, Michaliszyn SF, Lee S, Nasr A, and Arslanian S. Anti-Mullerian Hormone in Obese Adolescent Girls With Polycystic Ovary Syndrome. J Adolesc Health 2017; 60(3): 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]


