Fig. 1. Nsp1 protein of SARS-CoV-2 interacts with the mRNA nuclear export receptor NXF1.
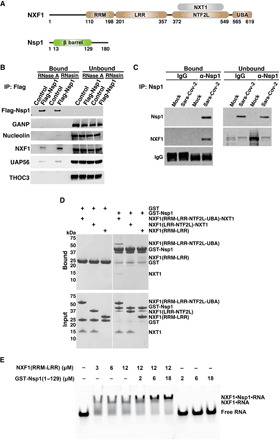
(A) Schematic representation of NXF1-NXT1 and Nsp1. NXF1 contains an N-terminal RNA recognition motif (RRM), a leucine-rich repeat domain (LRR), a nuclear transport factor 2-like domain (NTF2L), and a ubiquitin-associated domain (UBA). RRM and LRR domains form an RNA binding module. NXT1 forms a heterodimer with NXF1 by binding to the NTF2L domain of NXF1. Nsp1 contains a β barrel domain. (B) 293T cells were transfected with 3xFlag-Nsp1 for 24 hours and then subjected to fractionation into nuclear and cytoplasmic fractions. See fig. S1 for fractionation controls. Nuclear lysates were subjected to immunoprecipitation (IP), followed by Western blot analysis to detect the indicated proteins. n = 3. (C) 293T cells expressing the SARS-CoV-2 receptor ACE2 protein were infected with SARS-CoV-2 at an MOI of 1 for 24 hours. Nsp1 was specifically immunoprecipitated from the cell lysates, and NXF1 interaction was detected by Western blot analysis. n = 3. (D) In vitro GST pull-down assays using the depicted purified recombinant proteins show that Nsp1 directly binds to NXF1. n = 3. (E) NXF1 associates with RNA in the presence of Nsp1. An electrophoretic mobility assay was carried out with a fluorescently labeled poly(U) 15-mer RNA and purified recombinant NXF1 (RRM-LRR) and/or Nsp1 (1-129) as indicated. n = 3.
