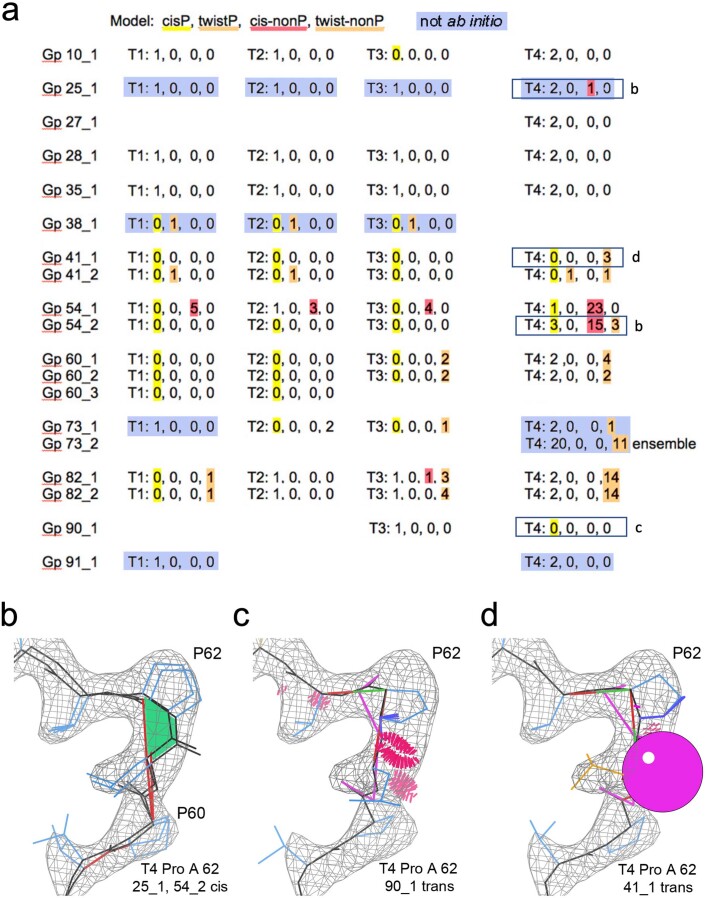
Extended Data Fig. 1. Evaluation of peptide bond geometry.
All 63 Challenge models were evaluated using MolProbity. APOF and ADH each have one cis peptide bond per subunit before a proline residue. (a) Counts of peptide bonds with each of the following conformational properties: cisP: cis peptide before proline, twistP: non-planar peptide (>30°) before proline, cis-nonP: cis peptide before non-proline, twist-nonP: non-planar peptide bond before non-proline. Incorrect cis-nonP usually occurred where the model was misfit (see Extended Data Figs. 2 and 3), while incorrect cis or trans Pro usually produced poor geometry. Values inconsistent with reference models are highlighted. Statistically, 1 in 20 proline residues are genuinely cis; only 1 in 3000 non-proline residues are genuinely cis, and strongly non-planar peptide bonds (>30°) are almost never genuine28. Models are identified by the submitting group (Gp #, group id as defined in Table 1), model number (some groups submitted multiple models), and Target (T1-T3: APOF, T4: ADH). Optimized models are shaded blue. Only two groups (28, 31) had all peptides correct for all 4 targets. Models illustrated in panels b-d are indicated by labeled boxes. (b) Correct cis peptide geometry for Pro A62 in two ADH models. (c) Incorrect trans peptide geometry, with huge clashes up to 1.25 Å overlap (clusters of hot pink spikes), 2 CaBLAM outliers (magenta CO dihedral lines), and poor density fit. (d) Incorrect trans peptide geometry, with huge 1.9 Å Cβ deviation at Leu 61 (magenta ball) because of incorrect hand of Cα, and 2 CaBLAM outliers. Molecular graphics were generated using KiNG.