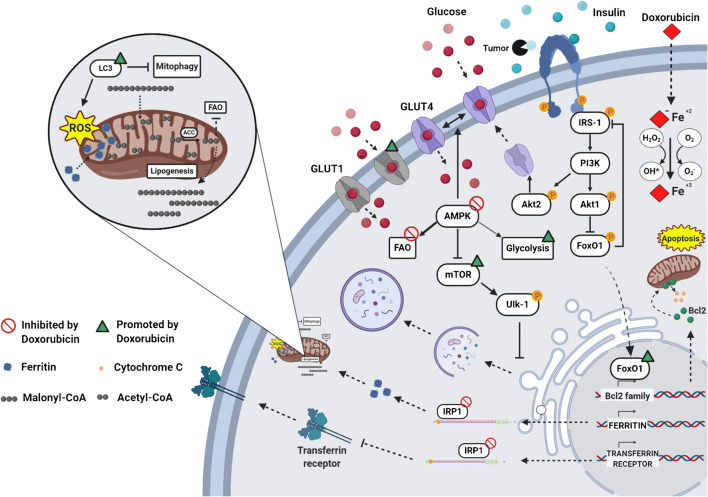
Fig. 1.
Metabolic changes induced by DOXO in cardiomyocytes. DOXO interferes with Fe2+ metabolism, leading to activation of ferroptosis through ROS production, disruption of IRP-1 activity, and iron accumulation into mitochondria. These events are hallmarks of mitochondrial dysfunction that leads to a block of fatty acid oxidation (FAO) and an increase in glycolysis, as a consequence of AMPK inhibition. Acetyl-CoA carboxylase (ACC), a direct downstream target inhibited by AMPK, is overactivated and catalyzes the formation of Malonyl-CoA, blocking FAO irreversibly. At the plasma membrane, DOXO promotes glucose uptake via GLUT4 through insulin-mediated activation of AMPK and AKT2. In addition, DOXO increases the expression of GLUT1, an insulin-independent glucose transporter, normally absent in the adult heart. Following the insulin desensitization induced by tumor-secreted factors, AKT1 signaling is disrupted and promotes FOXO1 nuclear translocation, inducing the activation of the apoptotic pathway through the expression of pro-apoptotic members of the Bcl-2 family. Finally, DOXO cardiotoxicity has been linked to autophagy dysregulation. DOXO inhibits autophagy by activating mTOR or by blocking AMPK, resulting in accumulation of undegraded autophagosomes and mitochondrial dysfunction with increased production of ROS. This figure was created with BioRender.com.