Figure 5. CXCL11 is differentially produced by B-cell subsets.
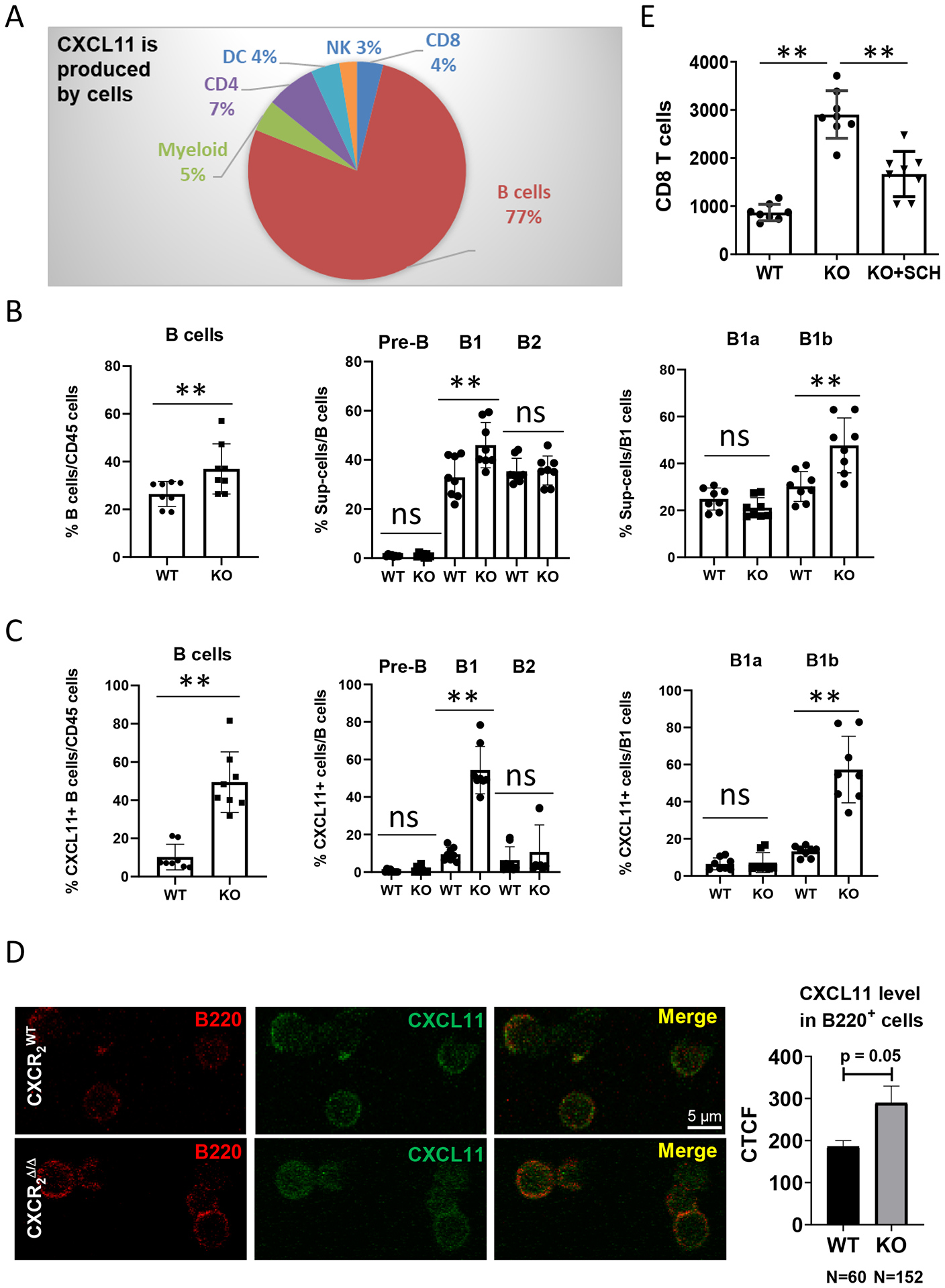
1×106 PyMT breast cancer cells were intravenously injected into CXCR2myeΔ/Δ (n=8) or littermate CXCR2WT mice (n=8). Two weeks after injection, (A) intracellular CXCL11 expression in the indicated intratumoral CD45+ cell subsets was analyzed by flow cytometry. B, Frequencies of B-cell subsets in TME: B cells (CD11b-CD19+B220+); B1 cells (CD19+B220+CD43+); B2 cells (CD19+B220+CD43−); Pre-B cells (CD19+B220−CD43−); B1a cells (CD19+B220+CD5+); and B1b cells (CD19+B220+CD5−). n=8. C, Percentage of CXCL11-expressing subpopulations of B cells. n=8. D, Localization of CXCL11 in B220+ B cells. B cells were isolated from tumor tissues of either CXCR2WT or CXCR2myeΔ/Δ mice and stained for B220 and CXCL11. Expression was visualized through confocal microscopy. Representative micrographs of tumor B cells (red) and intracellular CXCL11 (green). Quantification as corrected total cell fluorescence (CTCF) was performed and are presented as the mean of CXCL11-expressing B220+ B cells. E, 1×106 PyMT breast cancer cells were intravenously injected into CXCR2myeΔ/Δ (KO, n=8) or littermate CXCR2WT mice (WT, n=8). One week after injection, CD8+ T cells and pan B cells were isolated from tumor-bearing lungs, cultured short-term (18h), and conditioned medium was collected. Isolated CD8+ T cells were analyzed for migration toward the B cell-conditioned medium using a Boyden Chamber chemotaxis assay as described in Methods. Shown: number of CD8+ T cells in response to B cell-conditioned medium, or from B cell-conditioned medium from CXCR2myeΔ/Δ mice treated with 20 nM of the CXCR3 antagonist, SCH546738. Data were analyzed using the two-sample t-test with unequal variances for panels (B-C) and using one-way ANOVA with post-hoc test for panel (E); **p<0.01. Experiments were repeated, and values represent mean±SD.
