Figure 5. The pentatherapy regimen has therapeutic benefits against larger MC38-CEA tumors and against additional cold tumor models LLC-CEA and 4T1.
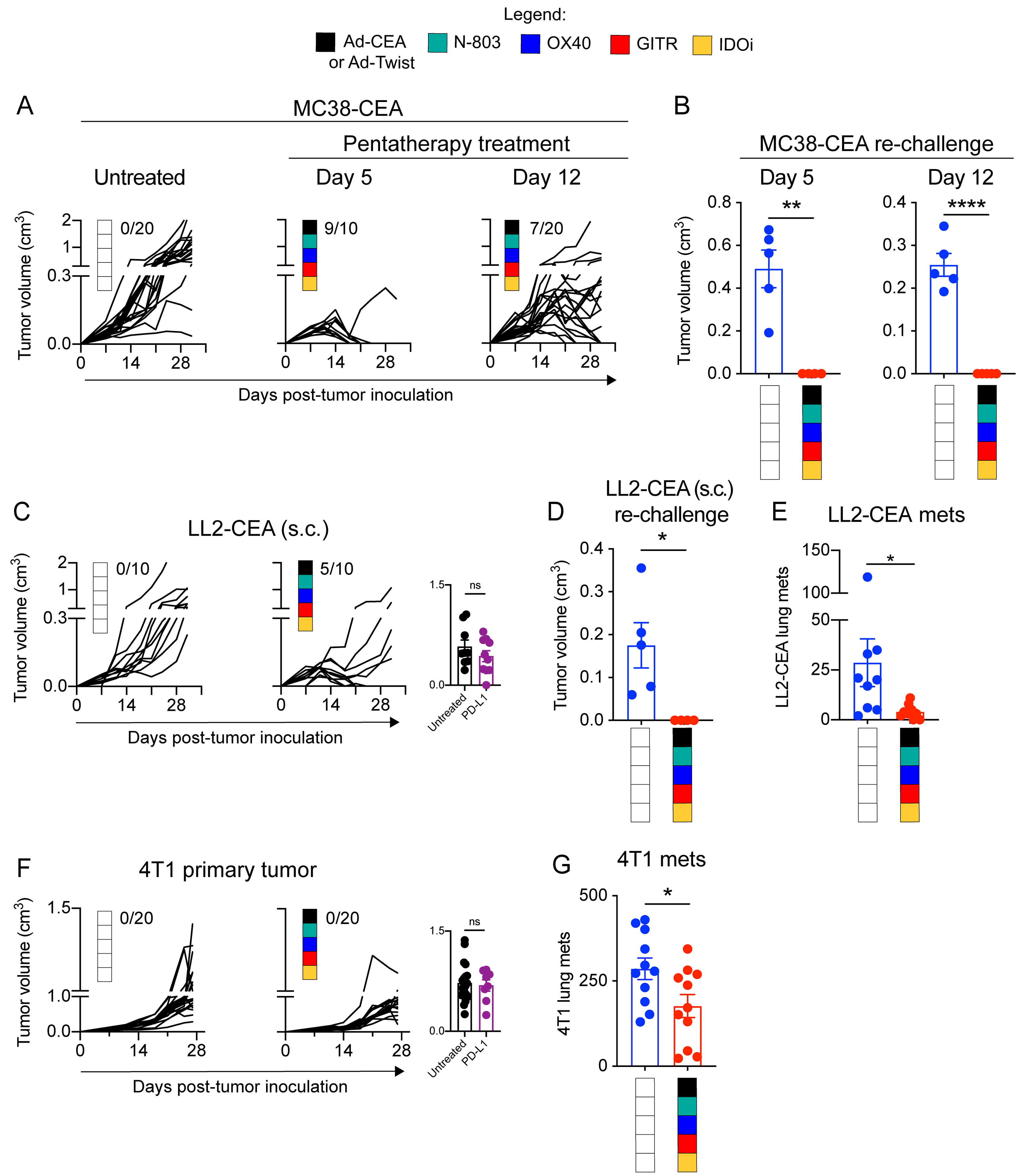
A. Female C57BL/6-CEA-Tg mice (8–16 weeks old; n=10–20/group) were inoculated with 3×105 MC38-CEA cells on the flank. The MC38-CEA tumor-bearing mice were treated with the pentatherapy regimen as described in Fig. 3A or given a delayed pentatherapy regimen treatment where Ad-CEA was given on days 14, 21 and 28 post-tumor inoculation, subcutaneous (s.c.); N-803 on days 21, and 28, s.c.; OX40 on days 14, 21 and 28, intraperitoneal (i.p.); (4) GITR on day 12, i.p.; and (5) the IDO inhibitor (IDOi) epacadostat feed starting at day 12. Tumor volume was monitored. Inset values represent fraction of mice that were cured in each group. B. Cured mice from (A) were re-challenged with MC38-CEA tumor cells (s.c.; 1×106 cells/mouse for the group that was treated using the original schedule, and 3×105 cells/mouse for the group that received delayed treatment), and tumor volume was reported on day 16 after tumor re-challenge. C. Female C57BL/6-CEA-Tg mice (8–16 weeks old; n = 10–20/group) were inoculated with 3×105 LL2-CEA cells on the flank (s.c.) and subsequently treated with the pentatherapy regimen as described in Fig. 3A. Tumor volume was monitored. Inset values represent fraction of mice that were cured in each group. D. Cured mice from (C) were re-challenged with 3×105 LL2-CEA tumor cells (s.c.), and tumor volume was reported on day 16 after tumor re-challenge. E. Female C57BL/6-CEA-Tg mice (8–16 weeks old, n=9–10/group) were injected with 5×105 LL2-CEA cells, intravenous (i.v.), and the animals were left untreated or administered with the pentatherapy combination as described in Fig. 3A. On day 28, lungs were collected, and metastatic tumor nodules were manually counted. F. Female Balb/c mice (8–16 weeks old) were inoculated with 5×104 4T1 cells on the mammary fat pad, s.c. 4T1-bearing mice (n=15/group) were either left untreated or administered with the pentatherapy combination, using Ad-Twist instead of Ad-CEA, as described in Fig. 3A. Primary tumor growth was monitored. Inset values represent fraction of mice that were cured in each group. G. On day 28 post-tumor inoculation, lung tissues were harvested from the 4T1-bearing animals. Clonogenic metastatic cell colonies were enumerated by culturing single-cell suspension of the lungs in a 6-thioguanine selection medium for at least 12 days. Two-way ANOVA with Tukey’s or Sidak’s post hoc test was used for grouped analyses; t-test was used to compare two groups. *P<0.05; **P<0.01; ****P<0.001. Error bars represent mean±SEM. These studies were repeated 3–4 times with similar results.
