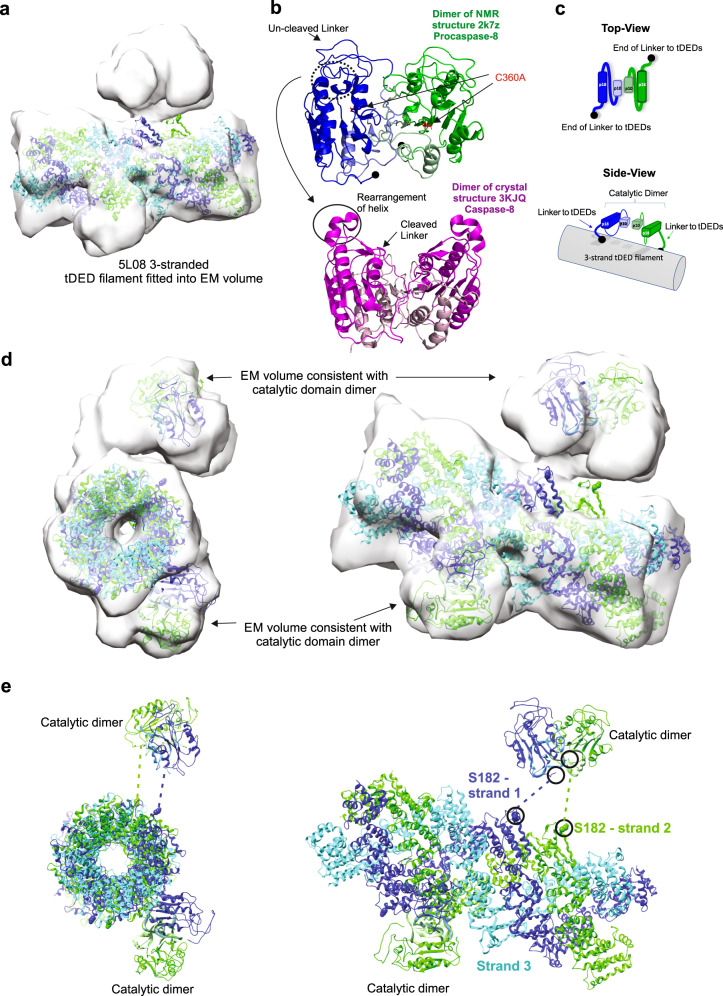
Fig. 3. Core tDED triple-helix facilitates inter-strand Caspase-8 catalytic domain anti-parallel dimerization.
Refined central region of full-length Caspase-8 filament with, a published tDED-only structure (5L08;12) fitted as a rigid body. b Ribbon NMR-based structural model of dimerized uncleaved catalytic subunit domains (2k7z; 21; upper panel, C360A in red and K224 as filled circle) and ribbon X-ray structure of cleaved catalytic subunit dimer (3KJQ;14; lower panel). c Schematic representation of uncleaved Caspase-8 p18 (large) and p10 (small) catalytic domains in anti-parallel orientation relative to tDED domains. d Fitted ribbon diagram of Caspase-8 tDEDs and catalytic domain assembly. Each strand of the helix is coloured; blue and green strands contain aligned DEDs, while in the cyan strand the DEDs are offset. e Caspase-8 catalytic domain subunit colours reflect the DED strand from which they originate. Distance between the termini connected by the dashed lines is approximately 35 Å, which can be accommodated by the 32 amino acid flexible linker between the tDED and catalytic domains of Caspase-8.