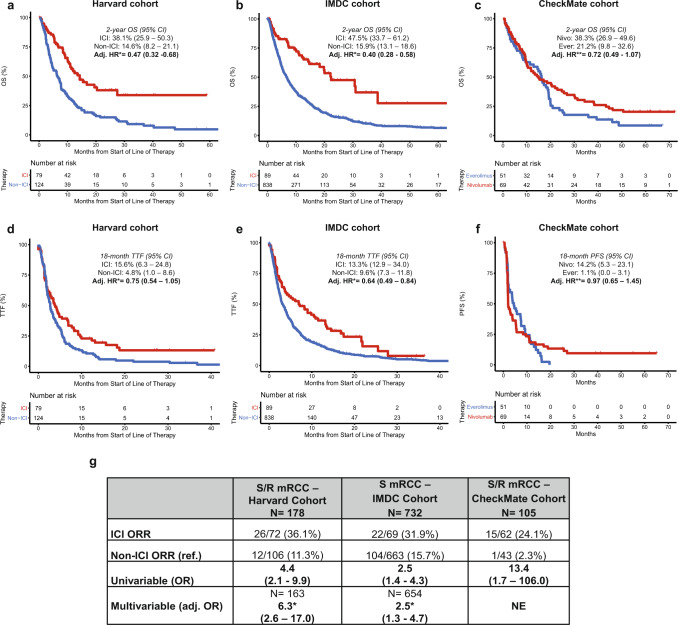
Fig. 3. Improved clinical outcomes of S/R RCC tumors on immune checkpoint inhibitors across clinical trial and real-word cohorts.
OS on ICI compared to non-ICI in the a Harvard, b IMDC and c CheckMate S/R RCC cohorts. TTF on ICI compared to non-ICI in the d Harvard and e IMDC S/R RCC cohorts and f PFS in the CheckMate S/R RCC cohort. g Summary table of overall response rate (among evaluable patients) on ICI compared to non-ICI in patients with S/R RCC across the Harvard, IMDC, and CheckMate cohorts. 95% CI: 95% Confidence Interval; Adj. Adjusted; Ever: Everolimus; HR: Hazard Ratio; ICI: Immune Checkpoint Inhibitor; IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; Nivo: Nivolumab; NE: Not Evaluable; OS: Overall Survival; S/R: Sarcomatoid/Rhabdoid. *Adjusted for IMDC (International Metastatic Renal Cell Carcinoma Database Consortium) risk groups, line of therapy, and background histology. **Adjusted for MSKCC (Memorial Sloan Kettering Cancer Center) risk groups.