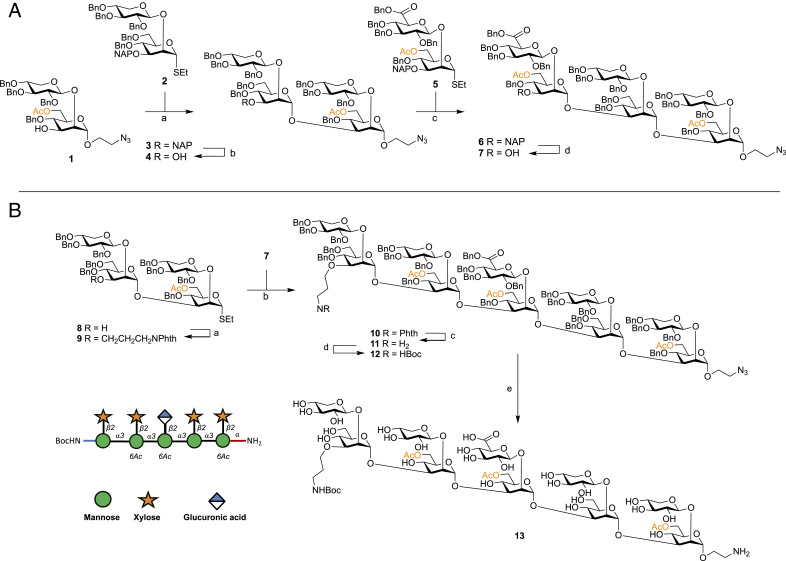
Fig. 2.
Synthesis of bifunctionally armed oligosaccharide. (A) Reagents and conditions: (a) DMTST, Et2O, 4 Å MS, 0 °C→room temperature, 80%; (b) DDQ, CH2Cl2:PBS (100 mM, pH 7.5) [85:15 (vol/vol)], 0 °C, 76%; (c) DMTST, Et2O, 4 Å MS, 0 °C→room temperature, 65%; (d) DDQ, CH2Cl2:PBS (100 mM, pH 7.5) [85:15 (vol/vol)], 0 °C, 70%. (B) Reagents and conditions: (a) NaH 60% dispersion in mineral oil, DMF, 4 Å MS, PhthNCH2CH2CH2Br, 0 °C→room temperature, 70%; (b) DMTST, Et2O, 4 Å MS, 0 °C→room temperature, 85%; (c) ethylene diamine, n-BuOH, 90 °C; (d) Boc2O, THF:H2O [70:30 (vol/vol)], 57% two steps; (f) Pd/C (pretreated catalyst) (28), H2, THF:tBuOH:PBS (100 mM, pH 5) [60:10:30 (vol/vol/v)], 66%.