Abstract
Simple Summary
One-third of adult acute myeloid leukemia (AML) harbors NPM1 mutations. A deep knowledge of the distribution of selected antigens on the surface of NPM1-mutated AML cells may help optimizing new therapies for this frequent AML subtype. CD123 is known to be expressed on leukemic cells but also on healthy hematopoietic and endothelial cells, although at lower levels. Differences in antigen densities between AML and healthy cells may enlighten therapeutic windows, where targeting CD123 could be effective without triggering “on-target off-tumor” toxicities. Here, we perform a thorough analysis of CD123 expression demonstrating high expression of this antigen on both NPM1-mutated bulk leukemic cells and CD34+CD38− cells.
Abstract
NPM1-mutated (NPM1mut) acute myeloid leukemia (AML) comprises about 30% of newly diagnosed AML in adults. Despite notable advances in the treatment of this frequent AML subtype, about 50% of NPM1mut AML patients treated with conventional treatment die due to disease progression. CD123 has been identified as potential target for immunotherapy in AML, and several anti-CD123 therapeutic approaches have been developed for AML resistant to conventional therapies. As this antigen has been previously reported to be expressed by NPM1mut cells, we performed a deep flow cytometry analysis of CD123 expression in a large cohort of NPM1mut and wild-type samples, examining the whole blastic population, as well as CD34+CD38− leukemic cells. We demonstrate that CD123 is highly expressed on NPM1mut cells, with particularly high expression levels showed by CD34+CD38− leukemic cells. Additionally, CD123 expression was further enhanced by FLT3 mutations, which frequently co-occur with NPM1 mutations. Our results identify NPM1-mutated and particularly NPM1/FLT3 double-mutated AML as disease subsets that may benefit from anti-CD123 targeted therapies.
Keywords: acute myeloid leukemia (AML), CD123, NPM1, FLT3, immunotherapy
1. Introduction
Acute myeloid leukemia (AML) is an aggressive cancer of hematopoietic stem and progenitor cells (HSPCs) [1], affecting almost 20,000 individuals every year in Europe [2]. Despite AML prognosis has significantly improved in recent years, 60% to 70% of patients diagnosed with AML eventually die of leukemia [1]. Such poor prognosis is mainly due to the high incidence of AML relapse after conventional treatment [3].
A significant fraction of AML relapses is thought to be secondary to the persistence of residual leukemic cells, able to re-establish the full tumor bulk [4]. In this regard, previous works suggest that leukemic cells with a stem-like phenotype (leukemic stem cells; LSCs) tend to survive chemotherapy, playing a major role in AML relapse [5,6]. Indeed, larger LSC pools at diagnosis are predictive of chemoresistance and worse prognosis [7].
Novel approaches aimed to eradicate residual disease in AML are under development [8,9]. Particularly, novel immunotherapeutic strategies targeting surface antigens are under investigation [10], with the hope to replicate the remarkable results of anti-CD19 bispecific antibodies and CAR-T cells in B-acute lymphoblastic leukemia (B-ALL) [11,12]. However, a major challenge for the clinical applicability of immunotherapy in AML is target selection, as no antigen that is selectively expressed on AML cells has been identified so far [13].
CD123, the alfa-subunit of the interleukin-3 (IL-3) receptor, is an attractive target, which has been reported to be expressed by the majority of AML patients, both on bulk leukemic cells and CD34+CD38− putative LSCs [14,15]. However, CD123 expression on normal HSPCs and endothelial cells still represents a potential threat for “on-target off-tumor” effects, including myelosuppression and vascular toxicities, especially for powerful antigen-sensitive strategies such as Chimeric Antigen Receptor T (CAR-T) cells [16,17]. We believe that identifying AML subgroups with the highest CD123 expression on AML cells and putative LSCs may open therapeutic windows where anti-CD123 immunotherapy could be effective without causing major toxicities on CD123-positive vital tissues.
In the past years, several groups have investigated the landscape of CD123 expression in AML. However, the majority of studies have evaluated CD123 expression only on bulk and CD34+ cells [18,19], while CD34+CD38− cells have been analyzed only in a relatively small number of patients [20,21,22]. Moreover, whether CD123 expression is higher on CD34+CD38− than bulk AML cells and whether it correlates with specific risk categories is still controversial [15,20].
About one-third of adult AML patients harbor NPM1 mutations [23]. Specific clinical and pathologic features granted NPM1-mutated AML the designation as a distinct entity of the World Health Organization classification of hematopoietic tumors [24]. Although large clinical studies have demonstrated that NPM1-mutated AML has a relatively favorable prognosis, about 50% of patients eventually die due to relapse and disease progression [25,26]. The prognosis is even poorer when FLT3 internal tandem duplications (FLT3-ITD) coexist [27]. Interestingly, a previous work from our group suggested that NPM1mut putative LSC express CD123 [28]. Although several studies have described CD123 expression in large cohorts that included NPM1mut patients, no study had been designed to specifically analyze this AML subgroup or to compare CD123 fluorescence intensities between bulk AML cells and the CD34+CD38− population [13,15,19].
Here, we investigate CD123 expression in a large number of newly diagnosed AML, focusing on the correlation between CD123 expression and NPM1 mutational status, with the aim to explore whether NPM1mut AML could represent an entity that could particularly benefit from anti-CD123 therapies.
2. Results
2.1. Study Population and Analysis
Between October 2010 and October 2020, 151 samples (74 bone marrows and 77 peripheral blood) from 80 female and 71 male adult patients with newly diagnosed AML (median age at diagnosis 60, range 22–90) were studied.
CD123 expression was studied by multiparameter flow cytometry on bulk leukemic cells in all samples. Additionally, we also analyzed CD123 levels on CD34+CD38− putative LCS in 115 samples containing at least 50 events in this rare subpopulation. CD123 expression levels were reported as the percent of positive cells (PPC) and as the median fluorescence intensity (MFI). PPC was available for all 151 samples, while MFI for 123 samples (see Methods). An arbitrary cut-off of 20% PPC was set to assign CD123 positivity. A summary of all results is presented in Table 1.
Table 1.
Summary of CD123 expression in all samples. Summary of CD123 expression studied by flow cytometry in bulk acute, myeloid leukiemia (AML) and CD34+CD38− cells. * CD123 expression was studied in immature cells gated as CD45dimSSClow cells (see Methods). LR, low risk; IR, intermediate risk; and HR, high risk. p-values are for unpaired t-tests, unless otherwise specified.
| Subgroup | Bulk Cells CD123 PPC Median (25–75th Percentile), n | Bulk Cells CD123 MFI Median (25–75th Percentile), n | CD34posCD38neg CD123 PPC Median (25–75th Percentile), n | CD34posCD38neg CD123 MFI Median (25–75th Percentile), n |
|---|---|---|---|---|
| All samples | 76 (48–91), 151 | 23.5 (13.7–46.3), 122 | 71 (41–95), 119 | 20 (9.7–66.1), 96 |
| Female | 78 (53.8–92), 80 | 29.1 (14.9–47.4), 68 | 84 (44.8–98), 60 | 30 (11.3–90), 52 |
| Male | 71 (46–86), 71 | 22.3 (13.4–38.6), 54 | 58 (35–91), 59 | 14.6 (9.6–36.6), 44 |
| p value | 0.2557 | 0.2883 | 0.0598 | 0.0201 |
| LR cytogenetics | 50.5 (41–61.8), 10 | 11.8 (11.8–25.5), 9 | ||
| IR cytogenetics | 80 (49.5–93), 81 | 23.7 (14.3–51.6), 65 | ||
| HR cytogenetics | 60 (27–80.3), 20 | 16 (10.5–35.3), 17 | ||
| p value (ANOVA) | 0.0286 | 0.0455 | ||
| NPM1mut | 84.5 (74.3–94), 68 | 39.8 (22.4–61), 54 | 94.5 (48.5–99), 54 | 47.4 (9.6–106.9), 45 |
| NPM1wt | 58 (32–79), 83 | 16.5 (10.7–28.4), 68 | 60 (36–85), 65 | 15 (9.9–29), 50 |
| p value | <0.0001 | <0.0001 | 0.0066 | <0.0001 |
| FLT3mut | 91.5 (75.3–95), 36 | 43.8 (19.7–68.4), 26 | 93 (58.5–98.8), 32 | 37.5 (9.7–103.7), 23 |
| FLT3wt | 66.5 (38.3–82.3), 86 | 20.7 (11.8–32.5), 67 | 61.1 (35.8–90.3), 62 | 16.9 (11–41.8), 47 |
| p value | <0.0001 | <0.0001 | 0.013 | 0.0483 |
| NPM1mut/FLT3-ITD | 93 (82.5–96), 21 | 61 (28.9–75), 15 | 98 (89.5–99), 21 | 90.6 (44.7–169.5), 14 |
| NPM1mut/FLT3wt | 81 (67.8–89.5), 29 | 28.5 (16.3–47.4), 22 | 63.5 (39.5–99), 18 | 16.9 (9.7–110), 15 |
| NPM1wt/FLT3-ITD | 83 (54.5–94.5), 13 | 27 (15.4–55.2) 10 | 63 (32.8–85.5), 10 | 10.3 (7.6–27.6) 7 |
| NPM1wt/FLT3wt | 53 (28–77), 57 | 14.6 (10.3–23.5), 45 | 61.1 (33–84.5), 44 | 18.6 (8.6–29.4), 33 |
| p value (ANOVA) | <0.0001 | <0.0001 | 0.0029 | <0.0001 |
| Healthy donors | 13 (5.25–16.25), 4 * | 15.5 (6–20.8), 4 * | 2.5 (1.3–8.3), 4 | 4.4 (1.9–9.5), 4 |
2.2. CD123 MFI Is Higher on Putative CD34+CD38− AML LSCs
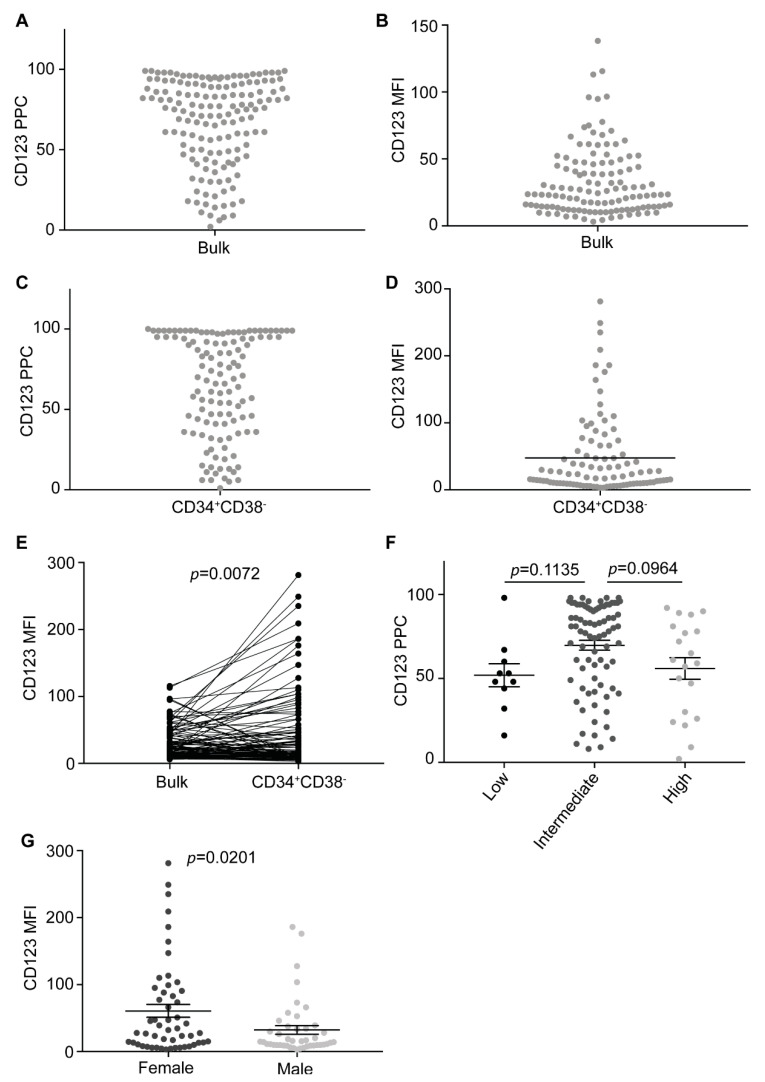
We first analyzed CD123 expression on bulk cells in all samples. As previously reported by others [14,15,18,19], the vast majority of cases resulted CD123-positive (138/151, 91%). However, variable expression levels were observed among positive cases (mean PPCs 73 ± 22 and mean MFI 35 ± 26) (Figure 1A,B). We then looked into CD34+CD38− cells, finding frequencies of CD123 positivity similar to those observed in bulk cells (mean PPCs 68 ± 30 and mean MFI 48 ± 60) (Figure 1C,D). Although no statistically significant difference was found between bulk and CD34+CD38− CD123 PPCs, CD123 MFI was higher in CD34+CD38− than in bulk cells (p = 0.0072) (Figure 1E), indicating that LSCs tend to have higher CD123 expression levels.
Figure 1.
CD123 is ubiquitously expressed in acute myeloid leukemia (AML). (A) CD123 percent of positive cells (PPCs) on bulk cells in all samples (n = 151). (B) CD123 mean fluorescence intensities (MFIs) on bulk cells in all samples available (n = 122). (C) CD123 PPCs on CD34+CD38− cells in all samples available (n = 119). (D) CD123 MFIs on CD34+CD38− cells in all samples available (n = 96). (E) CD123 MFIs on bulk cells compared to CD34+CD38− cells at diagnosis in all samples available (n = 96). Paired t-test. (F) CD123 PPCs on bulk cells sorted based on the cytogenetic risk (n = 111). Cytogenetics characteristics of all patients are reported in Table S1. Multiple comparison test. (G) CD123 MFIs on CD34+CD38− cells in female and male patients (n = 96). Unpaired t-test. Bars represent mean and standard error.
No statistically significant differences were found according to the cytogenetic risk stratification [27]. However, a clear trend towards higher CD123 expression levels was detected in the intermediate risk group (Figure 1F). Surprisingly, we also found a clear trend towards higher CD123 expression levels (MFI p = 0.00181 and PPC p = 0.0938) in female patients as compared to males when analyzing CD34+CD38− LSC (Figure 1G).
2.3. CD123 Expression Is Consistently High in NPM1mut AML LSCs
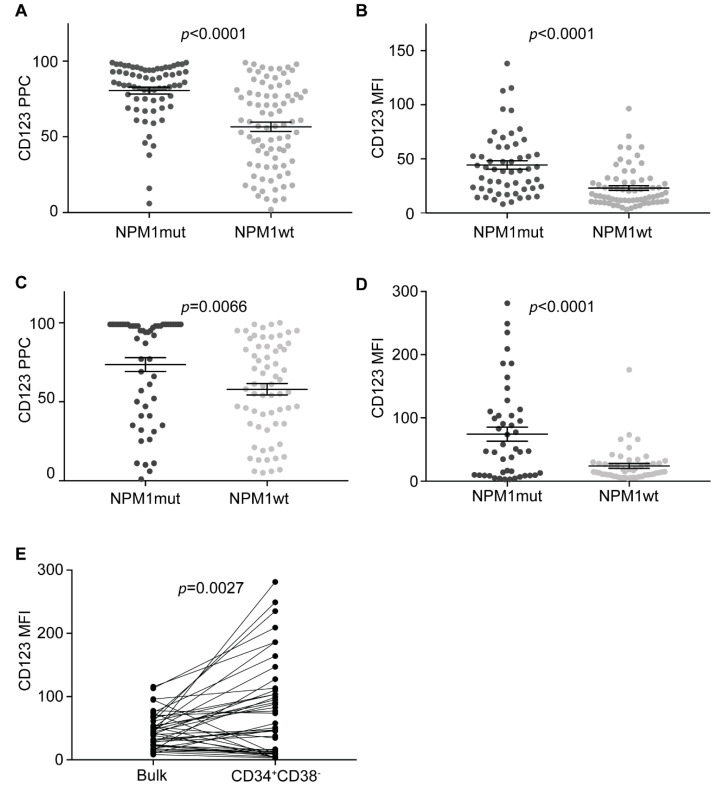
As previous xenograft experiments suggest that NPM1mut AML LSCs are CD123-positive [28], we investigated CD123 expression in NPM1mut samples. NPM1 was mutated in 68/151 patients (45%), and 97% (66/68) NPM1mut samples were CD123-positive. When compared with NPM1wt samples, NPM1mut cases displayed significantly higher CD123 expression levels on bulk AML cells (PPC and MFI p < 0.0001) (Figure 2A,B).
Figure 2.
CD123 is highly expressed on NPM1mut AML cells. (A) CD123 PPCs on NPM1mut and NPM1wt AML bulk cells in all samples (n = 151). Unpaired t-test. (B) CD123 MFIs on NPM1mut and NPM1wt AML bulk cells in all samples available (n = 122). Unpaired t-test. (C) CD123 PPCs on NPM1mut and NPM1wt CD34+CD38− cells in all samples available (n = 119). Unpaired t-test. (D) CD123 MFIs on NPM1mut and NPM1wt CD34+CD38− cells in all samples available (n = 96). Unpaired t-test. (E) CD123 MFIs on NPM1mut bulk AML cells compared to CD34+CD38− cells at diagnosis in all samples available (n = 44). Paired t-test. Bars represent mean and standard error.
We then looked at CD123 expression in NPM1mut CD34+CD38− AML putative LSCs. CD123 was positive in 91% of NPM1mut samples (49/54). Both PPCs and MFIs were significantly higher in NPM1mut putative LSC compared to NPM1wt samples (PPCs p = 0.0066 and MFI p < 0.0001) (Figure 2C,D). Average CD123 MFI was higher in CD34+CD38− putative LSC than in bulk NPM1mut AML cells (p = 0.0029), confirming that CD123 is highly expressed on the surface of NPM1mut AML LSCs (Figure 2E).
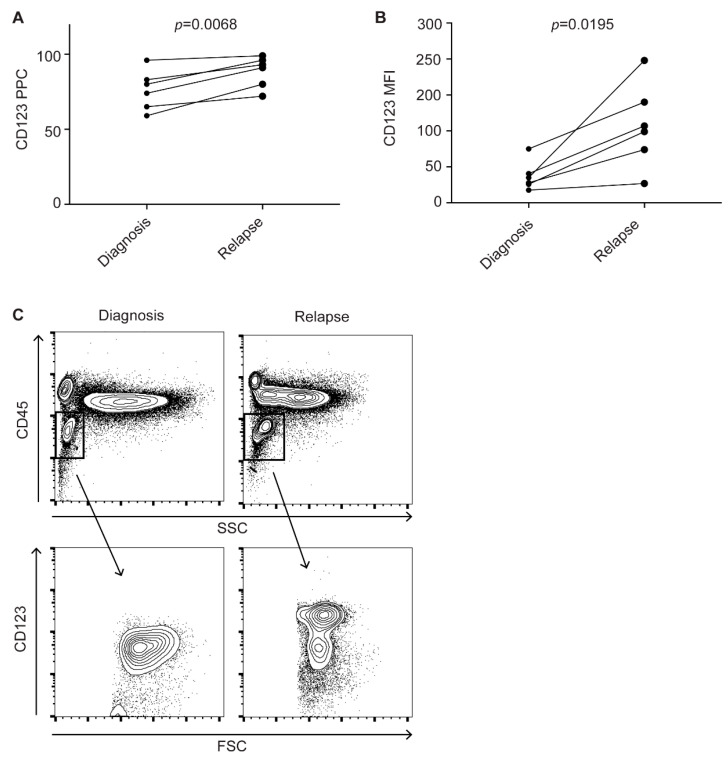
As several studies suggest that relapse frequently derives from residual LSCs after treatment [4,5], we analyzed CD123 expression on bulk AML cells in six NPM1mut patients at diagnosis and relapse. In all patients, CD123 expression increased at relapse, compared to diagnosis (PPC p = 0.0068 and MFI p = 0.0195) (Figure 3A–C). As two out of six patients had too-small numbers of CD34+CD38− cells at diagnosis (<50 events), no comparison between diagnosis and relapse was possible in this subpopulation. Nonetheless, all six samples showed very high CD123 expression levels on CD34+CD38− cell at relapse (average PPC 96 and average MFI 139). Altogether, these data confirm that CD123 is consistently expressed on NPM1mut AML putative LSCs.
Figure 3.
CD123 expression increases in relapsed NPM1mut AML. (A) CD123 PPCs on bulk NPM1mut AML cells at diagnosis and relapse (n = 6). Paired t-test. (B) CD123 MFIs on bulk NPM1mut AML cells at diagnosis and relapse (n = 6). Paired t-test. (C) Flow cytometry dot plots showing an increase of CD123 expression at relapse (right plots) compared to diagnosis (left plots) in a representative case of NPM1mut AML.
2.4. CD123 Is Highly Expressed on FLT3-Mutated AML
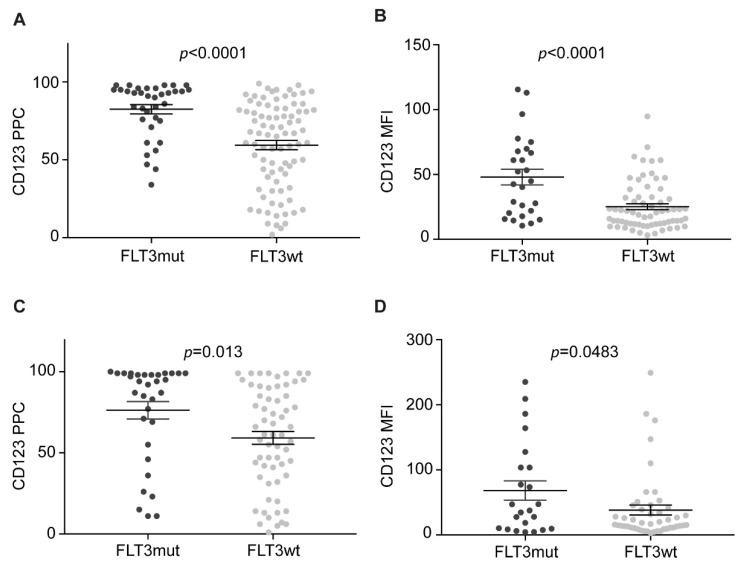
Since AML with mutated NPM1 frequently harbors FLT3 mutations, which have been previously reported to be associated with higher CD123 expression [29,30], we also analyzed the FLT3 mutational status in 122 samples. FLT3 was mutated in 46% (24/52) NPM1mut and in 19% (13/70) of NPM1wt cases. Two patients were positive for the FLT3-D835 mutation, while all others harbored FLT3-ITD. We first compared CD123 in FLT3-mutated (FLT3mut) and FLT3 wild-type (FLT3wt) samples. FLT3mut samples showed significantly higher CD123 levels than FLT3wt cases. This was true for bulk cells (PPC p < 0.0001 and MFI p < 0.0001) and, though with a lesser degree of certainty, for CD34+CD38− cells (PPC p = 0.013 and MFI p = 0.0483) (Figure 4A–D).
Figure 4.
CD123 expression on FLT3mut and FLT3wt AML cells. (A) CD123 PPCs on FLT3mut and FLT3wt AML bulk cells in all samples available (n = 112). Unpaired t-test. (B) CD123 MFIs on FLT3mut and FLT3wt AML bulk cells in all samples available (n = 93). Unpaired t-test. (C) CD123 PPCs on FLT3mut and FLT3wt CD34+CD38− cells in all samples available (n = 94). Unpaired t-test. (D) CD123 MFIs on FLT3mut and FLT3wt CD34+CD38− cells in all samples available (n = 70). Unpaired t-test. Bars represent mean and standard error.
2.5. NPM1 and FLT3 Mutations Cooperate in Promoting CD123 Expression
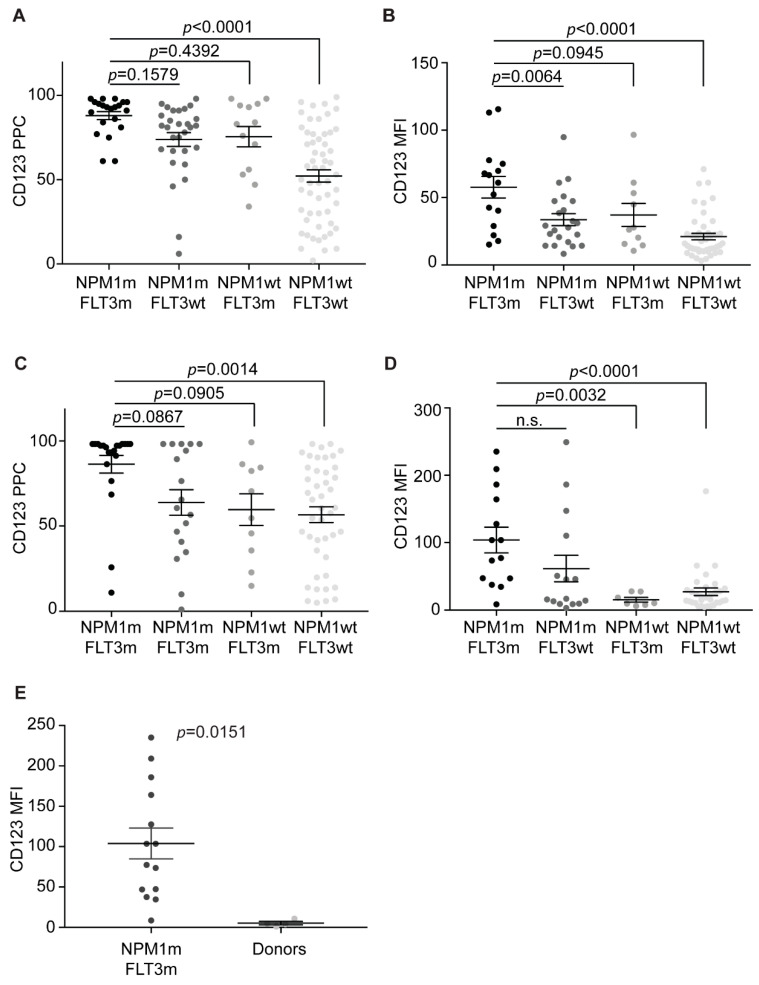
We then analyzed the CD123 expression in each of four the different NPM1/FLT3-ITD genotypes. The NPM1mut/FLT3-ITD, NPM1mut/FLT3wt, NPM1wt/FLT3-ITD, and NPM1wt/FLT3wt genotypes accounted, respectively, for 21/120 (18%), 29/120 (24%), 13/120 (11%), and 57/120 (47%). The highest CD123 expression was detected in double-mutated patients, while NPM1wt/FLT3wt cases displayed the lowest expression levels (Figure 5A,B), and NPM1mut/FLT3wt and NPM1wt/FLT3-ITD patients had intermediate CD123 (Figure 5A,B). Similar results were obtained studying either PPCs or MFIs in either bulk cells or in CD34+CD38− subpopulations (Figure 5C,D). These results strongly suggest that NPM1 mutations and FLT3-ITD cooperates in promoting CD123 expression.
Figure 5.
NPM1mut/FLT3-ITD AML LSC express the highest CD123 levels. (A) CD123 PPC on bulk AML cells, according to the NPM1 and FLT3 mutational status. Multiple comparisons test. (B) CD123 MFI on bulk AML cells, according to the NPM1 and FLT3 mutational status. Multiple comparison test. (C) CD123 PPC on CD34+CD38− AML cells, according to the NPM1 and FLT3 mutational status. Multiple comparison test. (D) CD123 MFI on CD34+CD38− AML cells, according to the NPM1 and FLT3 mutational status. Multiple comparison test. (E) CD123 MFI on NPM1mut/FLT3-ITD CD34+CD38− AML cells and CD34+CD38− cells from healthy donors. Unpaired t-test. NPM1m, NPM1mut. FLT3m, FLT3-ITD. Bars represent mean and standard error.
2.6. CD123 Expression on HSPCs Is Significantly Lower than NPM1mut/FLT3-ITD CD34+CD38− Cells
To compare the CD123 expression on LSC to that of normal progenitors, we analyzed the CD123 expression on bone marrow immature cells and on CD34+CD38− cells from four healthy donors. Overall, healthy immature cells displayed very low CD123 levels (Table 1). Specifically, CD45dim-SSClow cells showed a median value of CD123 PPCs of 12 and a median value of MFIs of 15, while CD34+CD38− cells displayed even lower CD123 levels, with median values of CD123 PPC and MFI, respectively, of 3 and 4. With the caveat of the small sample size, we compared CD123 expression on CD34+CD38− cells from healthy donors with CD34+CD38− LSCs. As anticipated, the most significant difference was found with NPM1mut/FLT3-ITD CD34+CD38− leukemic cells (MFI p = 0.0151) (Figure 5E), further confirming very high CD123 expression in this AML subset.
3. Discussion
Immunotherapy targeting antigens expressed on AML cells represents a promising approach with the potential to reproduce the outstanding results achieved in B-ALL [31]. Despite several preclinical studies demonstrating the powerful antileukemic effects of bispecific antibodies and CAR-T cells targeting single AML antigens [32,33,34], only a few clinical trials are currently underway in AML patients [35]. Such a slow translation into clinical success is mainly due to the absence of targets with optimal expression profiles (i.e., highly expressed on neoplastic cells with low or no expression on vital healthy tissues) [10,13].
CD123 shows several features that prioritize this antigen among all promising potential target in AML. Previous studies have shown that the majority of AML patients express CD123 [13,15,18,19,20,22] and that higher CD123 expression seems to be associated with poorer prognosis [36,37,38]. Consequently, targeting CD123 represents an attractive approach to improve outcomes in AML.
However, CD123 expression on HSPCs and endothelium still represents a major obstacle [32]. Although CD123 expression on endothelial cells is very low [39], high levels of interferon-γ and tumor necrosis factor-α induced by cytokine release syndrome may facilitate CD123 expression and trigger capillary leak syndrome (CLS) [40]. This mechanism could explain why CLS occurred in two patients enrolled in a phase 1 first-in-human trial (NCT03203369 and NCT03190278) exploring the safety of a “universal” anti-CD123 CAR-T cell [41].
CD123 expression density is higher on AML cells than normal tissues. An important challenge is to find an optimal strategy where anti-CD123 CAR-T cells can still efficiently recognize leukemic cells while avoiding capillary leak syndrome and prolonged myelosuppression. Stevens et al. recently showed, by epitope density analysis, that anti-CD123 CAR-T cells could be able to eliminate tumor cells with higher CD123 antigen density, while sparing normal hematopoietic stem cells with lower antigen densities [42]. Arcangeli et al. confirmed the importance of CD123 antigen density and CAR affinity to fine-tune the level of anti-CD123 reactivity, merging efficacy with safety. In their study, the CAR-binding affinity was lowered in order to reduce the cytotoxicity against CD123low endothelial cell lines while holding high activity against CD123high AML cells lines [43]. In this context, identifying AML subsets with the highest CD123 expression levels seems relevant for the design of future clinical studies.
In this study, we demonstrate that CD123 is highly expressed on NPM1mut AML cells. CD123 expression on NPM1mut cells was already reported by our group while characterizing NPM1mut CD34+CD38− leukemia-initiating cells in xenograft experiments [28]. Here, we show consistently high CD123 levels on putative NPM1mut CD34+CD38− cells in patients, suggesting that anti-CD123 immunotherapies could be particularly effective in NPM1mut AML. The molecular mechanisms behind the higher CD123 expression and NPM1 mutations in AML are unclear. However, NPM1 seems to play an important role in the differentiation of myeloid progenitors into mature myeloid cells through the IL-3/CD123/JAK2/STAT5 pathway [44,45,46]. Therefore, the interplay between NPM1 mutation and high CD123 expression warrants further investigations.
CD123 expression was found to be particularly high in NPM1mut/FLT3-ITD samples. This was observed both on bulk cells, as well as in CD34+CD38− cells. As NPM1mut/FLT3-ITD AML patients have relatively poor outcomes due to high relapse rates [26], we envision that anti-CD123 immunotherapy could be of particular benefit for this specific subset of patients.
We also found consistently higher CD123 levels at relapse when comparing CD123 expression in NPM1mut AML at diagnosis and relapse. We therefore hypothesize that CD123 plays a major role in the maintenance of this AML entity, given the importance of the IL-3 receptor for stem cell biology. This hypothesis is in line with several studies demonstrating the engraftment failure of CD123-negative AML-LSCs [20,47,48]. These results also suggest that CD123 may be a useful surface marker for measurable residual disease (MRD) monitoring by multiparameter flow cytometry. In this regard, the European LeukemiaNet recommends including CD123 while assessing MRD to identify early progenitors and LCSs [49]. Future studies will address the impact of CD123 in MRD monitoring of NPM1mut AML.
In conclusion, this work demonstrates that CD123 is highly expressed on the surface of NPM1mut AML cells both at diagnosis and relapse, with the highest levels detected in NPM1mut/FLT3-ITD samples. This study establishes NPM1mut AML as an attractive candidate for CD123-targeted therapeutic strategies laying the groundwork for the design of clinical trials in this specific AML subset.
4. Materials and Methods
4.1. Samples from AML Patients and Healthy Donors
BMs and PBs were collected in seven different Italian centers and subsequently analyzed in our laboratory within 24 h from sampling. In 6 cases, paired BM or PB samples were collected at both diagnosis and relapse. To compare CD123 expression in normal and pathologic samples, we also studied 4 bone marrows from healthy donors. All patients and donors gave their written informed consent before either BM aspiration or PB collection.
Routine morphologic, immunohistochemistry, cytogenetic, and molecular studies to assess NPM1 and FLT3 mutational status were performed as previously described [28]. NPM1 mutations were also confirmed by Western blot analysis [50]. NPM1 and FLT3 mutational status was available, respectively, for 151/151 and 122/151 samples. In 111 cases, a successful karyotype analysis was obtained through standard G-banding [51]. Cytogenetic risk was defined according to the European LeukemiaNet 2017 recommendations, as previously reported [27]. Cytogenetics characteristics are reported in Supplementary Table S1.
4.2. Flow Cytometric Immunophenotyping
Heparinized peripheral blood or bone marrow samples were lysed for erythrocytes and stained with predefined optimal concentrations of 4 antibodies: CD34-FITC, CD123-PE, CD45-PerCP, and CD38-APC (Becton Dickinson, San Diego, CA, USA). Blasts were gated as CD45dim-side scatter (SSC)low cells in samples with no- or early myeloid differentiation (see, also, Figure 3C) and as CD45dim-pos-SSClow-int in cases with myelomonocytic or monocytic differentiation. CD123 expression analysis was analyzed on bulk leukemic cells and CD34+CD38− precursors.
To set the cut-off point to distinguish between CD123 negative and positive cells, we used the “Fluorescence Minus One” technique, as described by Perfetto et al. [52]. A single case was arbitrarily judged CD123-positive when the percentage of positive bulk cells was higher than 20%. In healthy controls, immature cells were selected as CD45dim-SSClow and CD123 analysis was performed on both whole immature cell population and CD34posCD38neg precursors. Data were reported as both CD123 percentage of positive cells (PPC) and median fluorescence intensity (MFI).
In all specimens, cell doublets and debris were excluded from analysis by forward scatter (FSC) vs. SSC dot-plot examination. Analysis was performed on either a FACSCalibur or FACSCanto II flow cytometers using the CellQuest Pro 6.0 or FACS Diva 7.0 analysis software (BD Biosciences, Franklin Lakes, NJ, USA). PPC was determined on all samples (analyzed on FACSCalibur or FACSCanto II). MFI was also studied in all samples; however, only those samples acquired on FACSCalibur were reported (i.e., the vast majority), as MFI values are not comparable between these two machines (four decades vs. five decades).
4.3. Statistical Analysis
Statistical analysis was carried out using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). Differences between two groups were determined using paired or unpaired two-tailed t-test, depending on the experiment performed (see figure legends for each individual experiment). Differences among three or more groups were compared with an unpaired ANOVA test performing post-hoc multiple comparison tests. In all tests, p-values were considered statistically significant if <0.05.
5. Conclusions
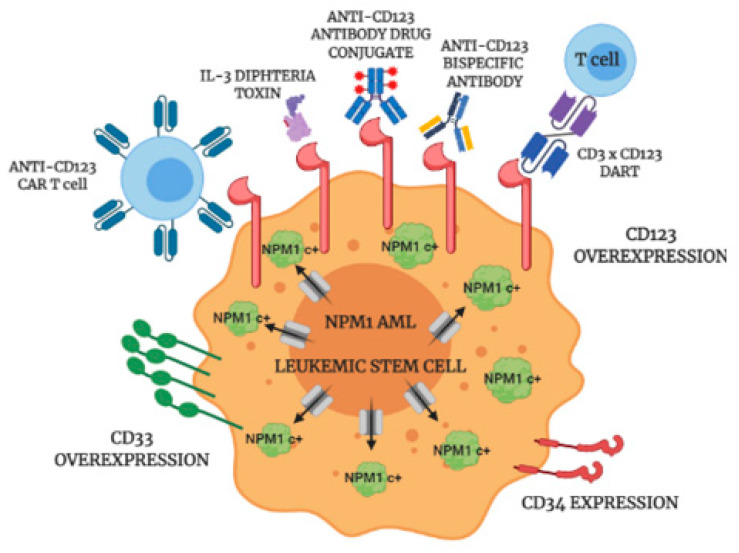
Our results suggest that NPM1mut AML could be particularly sensitive to anti-CD123 target therapies, for the differences in CD123 antigen densities between NPM1mut AML and healthy cells expressing low levels of CD123 (such as endothelial cells and HSCs) could reveal a therapeutic window where immunotherapeutic strategies could effectively eliminate NPM1mut LSCs (Figure 6) while avoiding capillary leak syndrome and prolonged pancytopenia. In conclusion, we believe that anti-CD123 immunotherapy holds promise for success in NPM1mut AML.
Figure 6.
Anti-CD123 targeted therapies. Cartoon depicting the possible approaches to target CD123 on NPM1mut AML LSCs. NPM1c+, mutant NPM1; DART, dual affinity retargeting.
Acknowledgments
Figure 6 was created on biorender.com.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/3/496/s1, Table S1: Karyotyping results of all patients enrolled in the study.
Author Contributions
Conceptualization, V.M.P., I.G., L.B., and M.P.M.; methodology, L.B., M.P.M.; formal analysis, V.M.P., I.G., R.R., L.B., and M.P.M.; resources:, F.M. (Francesca Milano), F.M. (Federica Mezzasoma), A.M., O.S., A.R., O.A., G.A., F.D.R., S.A. and B.F.; data curation, V.M.P., L.B., and M.P.M.; writing—original draft preparation, V.M.P.; writing—review and editing, L.B. and M.P.M.; supervision, L.B. and M.P.M.; and funding acquisition, L.B. and M.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Research Council (ERC), consolidator grant 2016 n. 725725, and the Associazione Italiana per la Ricerca sul Cancro (AIRC) Start-Up grant 2019, n. 22895
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study, as it was retrospective in nature and performed on routine diagnostic samples.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Döhner H., Weisdorf D.J., Bloomfield C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.Visser O., Trama A., Maynadié M., Stiller C., Marcos-Gragera R., De Angelis R., Mallone S., Tereanu C., Allemani C., Ricardi U., et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur. J. Cancer. 2012;48:3257–3266. doi: 10.1016/j.ejca.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Ganzel C., Sun Z., Cripe L.D., Fernandez H.F., Douer D., Rowe J.M., Paietta E.M., Ketterling R., O’Connell M.J., Wiernik P.H., et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am. J. Hematol. 2018;93:1074–1081. doi: 10.1002/ajh.25162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hope K.J., Jin L., Dick J.E. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat. Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 5.Jonas B.A. On the origin of relapse in AML. Sci. Transl. Med. 2017;9:eaan8205. doi: 10.1126/scitranslmed.aan8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roboz G.J., Guzman M. Acute myeloid leukemia stem cells: Seek and destroy. Expert Rev. Hematol. 2009;2:663–672. doi: 10.1586/ehm.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plesa A., Dumontet C., Mattei E., Tagoug I., Hayette S., Sujobert P., Tigaud I., Pages M.P., Chelghoum Y., Baracco F., et al. High frequency of CD34+CD38-/low immature leukemia cells is correlated with unfavorable prognosis in acute myeloid leukemia. World J. Stem Cells. 2017;9:227–234. doi: 10.4252/wjsc.v9.i12.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollyea D.A., Jordan C.T. Therapeutic targeting of acute myeloid leukemia stem cells. Blood. 2017;129:1627–1635. doi: 10.1182/blood-2016-10-696039. [DOI] [PubMed] [Google Scholar]
- 9.Arnone M., Konantz M., Hanns P., Paczulla Stanger A.M., Bertels S., Godavarthy P.S., Christopeit M., Lengerke C. Acute Myeloid Leukemia Stem Cells: The Challenges of Phenotypic Heterogeneity. Cancers. 2020;12:3742. doi: 10.3390/cancers12123742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anguille S., Van Tendeloo V.F., Berneman Z.N. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. 2012;26:2186–2196. doi: 10.1038/leu.2012.145. [DOI] [PubMed] [Google Scholar]
- 11.Mardiana S., Gill S. CAR T Cells for Acute Myeloid Leukemia: State of the Art and Future Directions. Front. Oncol. 2020;10:697. doi: 10.3389/fonc.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotiroti M.C., Arcangeli S., Casucci M., Perriello V., Bondanza A., Biondi A., Tettamanti S., Biagi E. Acute Myeloid Leukemia Targeting by Chimeric Antigen Receptor T Cells: Bridging the Gap from Preclinical Modeling to Human Studies. Hum. Gene Ther. 2017;28:231–241. doi: 10.1089/hum.2016.092. [DOI] [PubMed] [Google Scholar]
- 13.Haubner S., Perna F., Köhnke T., Schmidt C., Berman S., Augsberger C., Schnorfeil F.M., Krupka C., Lichtenegger F.S., Liu X., et al. Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leukemia. 2019;33:64–74. doi: 10.1038/s41375-018-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Testa U., Pelosi E., Castelli G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers. 2019;11:1358. doi: 10.3390/cancers11091358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bras A.E., de Haas V., van Stigt A., Jongen-Lavrencic M., Beverloo H.B., Te Marvelde J.G., Zwaan C.M., van Dongen J.J.M., Leusen J.H.W., van der Velden V.H.J. CD123 expression levels in 846 acute leukemia patients based on standardized immunophenotyping. Cytometry B. Clin. Cytom. 2019;96:134–142. doi: 10.1002/cyto.b.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taussig D.C., Pearce D.J., Simpson C., Rohatiner A.Z., Lister T.A., Kelly G., Luongo J.L., Danet-Desnoyers G.-A.H., Bonnet D. Hematopoietic stem cells express multiple myeloid markers: Implications for the origin and targeted therapy of acute myeloid leukemia. Blood. 2005;106:4086–4092. doi: 10.1182/blood-2005-03-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mardiros A., Forman S.J., Budde L.E. T cells expressing CD123 chimeric antigen receptors for treatment of acute myeloid leukemia. Curr. Opin. Hematol. 2015;22:484–488. doi: 10.1097/MOH.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testa U., Riccioni R., Militi S., Coccia E., Stellacci E., Samoggia P., Latagliata R., Mariani G., Rossini A., Battistini A., et al. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood. 2002;100:2980–2988. doi: 10.1182/blood-2002-03-0852. [DOI] [PubMed] [Google Scholar]
- 19.Ehninger A., Kramer M., Röllig C., Thiede C., Bornhäuser M., von Bonin M., Wermke M., Feldmann A., Bachmann M., Ehninger G., et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218. doi: 10.1038/bcj.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan C.T., Upchurch D., Szilvassy S.J., Guzman M.L., Howard D.S., Pettigrew A.L., Meyerrose T., Rossi R., Grimes B., Rizzieri D.A., et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 21.Xie L.H., Biondo M., Busfield S.J., Arruda A., Yang X., Vairo G., Minden M.D. CD123 target validation and preclinical evaluation of ADCC activity of anti-CD123 antibody CSL362 in combination with NKs from AML patients in remission. Blood Cancer J. 2017;7:e567. doi: 10.1038/bcj.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Mawali A., Pinto A.D., Al-Zadjali S. CD34+CD38-CD123+ Cells Are Present in Virtually All Acute Myeloid Leukaemia Blasts: A Promising Single Unique Phenotype for Minimal Residual Disease Detection. Acta Haematol. 2017;138:175–181. doi: 10.1159/000480448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falini B., Mecucci C., Tiacci E., Alcalay M., Rosati R., Pasqualucci L., La Starza R., Diverio D., Colombo E., Santucci A., et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 24.Falini B., Brunetti L., Sportoletti P., Martelli M.P. NPM1-mutated acute myeloid leukemia: From bench to bedside. Blood. 2020;136:1707–1721. doi: 10.1182/blood.2019004226. [DOI] [PubMed] [Google Scholar]
- 25.Straube J., Ling V.Y., Hill G.R., Lane S.W. The impact of age, NPM1mut, and FLT3ITD allelic ratio in patients with acute myeloid leukemia. Blood. 2018;131:1148–1153. doi: 10.1182/blood-2017-09-807438. [DOI] [PubMed] [Google Scholar]
- 26.Ostronoff F., Othus M., Lazenby M., Estey E., Appelbaum F.R., Evans A., Godwin J., Gilkes A., Kopecky K.J., Burnett A., et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: A SWOG and UK National Cancer Research Institute/Medical Research Council report. J. Clin. Oncol. 2015;33:1157–1164. doi: 10.1200/JCO.2014.58.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boddu P.C., Kadia T.M., Garcia-Manero G., Cortes J., Alfayez M., Borthakur G., Konopleva M., Jabbour E.J., Daver N.G., DiNardo C.D., et al. Validation of the 2017 European LeukemiaNet classification for acute myeloid leukemia with NPM1 and FLT3-internal tandem duplication genotypes. Cancer. 2019;125:1091–1100. doi: 10.1002/cncr.31885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martelli M.P., Pettirossi V., Thiede C., Bonifacio E., Mezzasoma F., Cecchini D., Pacini R., Tabarrini A., Ciurnelli R., Gionfriddo I., et al. CD34+ cells from AML with mutated NPM1 harbor cytoplasmic mutated nucleophosmin and generate leukemia in immunocompromised mice. Blood. 2010;116:3907–3922. doi: 10.1182/blood-2009-08-238899. [DOI] [PubMed] [Google Scholar]
- 29.Al-Mawali A., Gillis D., Lewis I. Immunoprofiling of leukemic stem cells CD34+/CD38-/CD123+ delineate FLT3/ITD-positive clones. J. Hematol. Oncol. 2016;9:61. doi: 10.1186/s13045-016-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelini D.F., Ottone T., Guerrera G., Lavorgna S., Cittadini M., Buccisano F., De Bardi M., Gargano F., Maurillo L., Divona M., et al. A Leukemia-Associated CD34/CD123/CD25/CD99+ Immunophenotype Identifies FLT3-Mutated Clones in Acute Myeloid Leukemia. Clin. Cancer Res. 2015;21:3977–3985. doi: 10.1158/1078-0432.CCR-14-3186. [DOI] [PubMed] [Google Scholar]
- 31.Slaney C.Y., Wang P., Darcy P.K., Kershaw M.H. CARs versus BiTEs: A Comparison between T Cell-Redirection Strategies for Cancer Treatment. Cancer Discov. 2018;8:924–934. doi: 10.1158/2159-8290.CD-18-0297. [DOI] [PubMed] [Google Scholar]
- 32.Gill S., Tasian S.K., Ruella M., Shestova O., Li Y., Porter D.L., Carroll M., Danet-Desnoyers G., Scholler J., Grupp S.A., et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casucci M., Nicolis di Robilant B., Falcone L., Camisa B., Norelli M., Genovese P., Gentner B., Gullotta F., Ponzoni M., Bernardi M., et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 34.Tettamanti S., Marin V., Pizzitola I., Magnani C.F., Giordano Attianese G.M.P., Cribioli E., Maltese F., Galimberti S., Lopez A.F., Biondi A., et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br. J. Haematol. 2013;161:389–401. doi: 10.1111/bjh.12282. [DOI] [PubMed] [Google Scholar]
- 35.Cummins K.D., Gill S. Will CAR T cell therapy have a role in AML? Promises and pitfalls. Semin. Hematol. 2019;56:155–163. doi: 10.1053/j.seminhematol.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Vergez F., Green A.S., Tamburini J., Sarry J.-E., Gaillard B., Cornillet-Lefebvre P., Pannetier M., Neyret A., Chapuis N., Ifrah N., et al. High levels of CD34+CD38low/-CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: A Groupe Ouest-Est des Leucemies Aigues et Maladies du Sang (GOELAMS) study. Haematologica. 2011;96:1792–1798. doi: 10.3324/haematol.2011.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vergez F., Nicolau-Travers M.-L., Bertoli S., Rieu J.-B., Tavitian S., Bories P., Luquet I., De Mas V., Largeaud L., Sarry A., et al. CD34+CD38-CD123+ Leukemic Stem Cell Frequency Predicts Outcome in Older Acute Myeloid Leukemia Patients Treated by Intensive Chemotherapy but Not Hypomethylating Agents. Cancers. 2020;12:1174. doi: 10.3390/cancers12051174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yabushita T., Satake H., Maruoka H., Morita M., Katoh D., Shimomura Y., Yoshioka S., Morimoto T., Ishikawa T. Expression of multiple leukemic stem cell markers is associated with poor prognosis in de novo acute myeloid leukemia. Leuk. Lymphoma. 2018;59:2144–2151. doi: 10.1080/10428194.2017.1410888. [DOI] [PubMed] [Google Scholar]
- 39.Korpelainen E.I., Gamble J.R., Vadas M.A., Lopez A.F. IL-3 receptor expression, regulation and function in cells of the vasculature. Immunol. Cell Biol. 1996;74:1–7. doi: 10.1038/icb.1996.1. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y., Wang S., Zhao L., Zhang B., Chen H. IFN-γ and TNF-α aggravate endothelial damage caused by CD123-targeted CAR T cell. Onco. Targets. Ther. 2019;12:4907–4925. doi: 10.2147/OTT.S205678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cellectis Cellectis Reports Clinical Hold of Ucart123 Studies. [(accessed on 20 January 2021)]; Available online: https://www.cellectis.com/en/press/cellectis-reports-clinical-hold-of-ucart123-studies.
- 42.Stevens B.M., Zhang W., Pollyea D.A., Winters A., Gutman J., Smith C., Budde E., Forman S.J., Jordan C.T., Purev E. CD123 CAR T cells for the treatment of myelodysplastic syndrome. Exp. Hematol. 2019;74:52–63.e3. doi: 10.1016/j.exphem.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arcangeli S., Rotiroti M.C., Bardelli M., Simonelli L., Magnani C.F., Biondi A., Biagi E., Tettamanti S., Varani L. Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia. Mol. Ther. 2017;25:1933–1945. doi: 10.1016/j.ymthe.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kieslinger M., Woldman I., Moriggl R., Hofmann J., Marine J.C., Ihle J.N., Beug H., Decker T. Antiapoptotic activity of Stat5 required during terminal stages of myeloid differentiation. Genes Dev. 2000;14:232–244. [PMC free article] [PubMed] [Google Scholar]
- 45.Ren Z., Aerts J.L., Vandenplas H., Wang J.A., Gorbenko O., Chen J.P., Giron P., Heirman C., Goyvaerts C., Zacksenhaus E., et al. Phosphorylated STAT5 regulates p53 expression via BRCA1/BARD1-NPM1 and MDM2. Cell Death Dis. 2016;7:e2560. doi: 10.1038/cddis.2016.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quelle F.W., Wang J., Feng J., Wang D., Cleveland J.L., Ihle J.N., Zambetti G.P. Cytokine rescue of p53-dependent apoptosis and cell cycle arrest is mediated by distinct Jak kinase signaling pathways. Genes Dev. 1998;12:1099–1107. doi: 10.1101/gad.12.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin L., Lee E.M., Ramshaw H.S., Busfield S.J., Peoppl A.G., Wilkinson L., Guthridge M.A., Thomas D., Barry E.F., Boyd A., et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Wittwer N.L., Brumatti G., Marchant C., Sandow J.J., Pudney M.K., Dottore M., D’Andrea R.J., Lopez A.F., Ekert P.G., Ramshaw H.S. High CD123 levels enhance proliferation in response to IL-3, but reduce chemotaxis by downregulating CXCR4 expression. Blood Adv. 2017;1:1067–1079. doi: 10.1182/bloodadvances.2016002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuurhuis G.J., Heuser M., Freeman S., Béné M.-C., Buccisano F., Cloos J., Grimwade D., Haferlach T., Hills R.K., Hourigan C.S., et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131:1275–1291. doi: 10.1182/blood-2017-09-801498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martelli M.P., Manes N., Liso A., Pettirossi V., Verducci Galletti B., Bigerna B., Pucciarini A., De Marco M.F., Pallotta M.T., Bolli N., et al. A western blot assay for detecting mutant nucleophosmin (NPM1) proteins in acute myeloid leukaemia. Leukemia. 2008;22:2285–2288. doi: 10.1038/leu.2008.149. [DOI] [PubMed] [Google Scholar]
- 51.Caspersson T., Zech L., Johansson C. Differential binding of alkylating fluorochromes in human chromosomes. Exp. Cell Res. 1970;60:315–319. doi: 10.1016/0014-4827(70)90523-9. [DOI] [PubMed] [Google Scholar]
- 52.Perfetto S.P., Chattopadhyay P.K., Roederer M. Seventeen-colour flow cytometry: Unravelling the immune system. Nat. Rev. Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request due to restrictions.