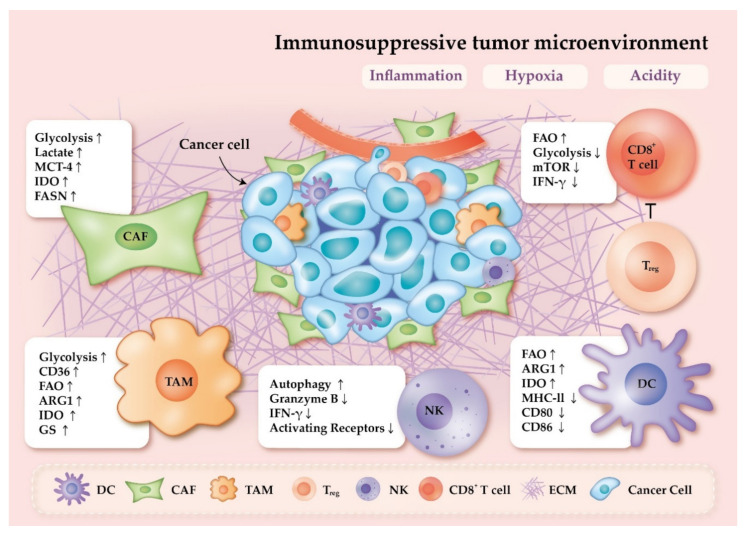
Figure 1.
Immune cells undergo metabolic reprogramming within the immunosuppressive tumor microenvironment (TME). Inflammation, hypoxia, and acidity are the three hallmarks in the TME, resulting in immunosuppression, cancer progression, and metastasis. Cancer associated fibroblasts (CAFs) are key players in generation and regulation of extracellular matrix (ECM). Stiffened ECM can promote glycolysis in CAFs and support cancer cells. Excessive production of lactate by CAFs can be transported via monocarboxylate transporter-4 (MCT-4) and leads to the acidification of the microenvironment. The expression of arginase 1 (ARG1) and indoleamine 2,3-dioxygenase (IDO) in tumor associated macrophages (TAMs) can contribute to the inhibition of effector T cells. TAMs can also accumulate lipid via scavenger receptor CD36 and serves as a source of fatty acid oxidation (FAO) used for differentiation and tumor promotion. Similar to TAMs, ARG1 and IDO expression are also upregulated in dendritic cells (DCs), which leads DCs toward a more immunosuppressive state. The decreased expression of major histocompatibility complex class II (MHC-II) could also impede antigen presentation by DCs and attenuate T cell-mediated immune responses in hypoxic conditions. The elevated activity and number in regulatory T cells (Tregs) may impede CD8+ T cells effector functions. Anti-cancer immunity property of CD8+ T cells and natural killer (NK) cells are attenuated by the dysregulation of metabolism in the TME. FASN, fatty acid synthase; mTOR, mammalian target of rapamycin; GS, glutamine synthetase; IFN-γ, interferon-gamma.