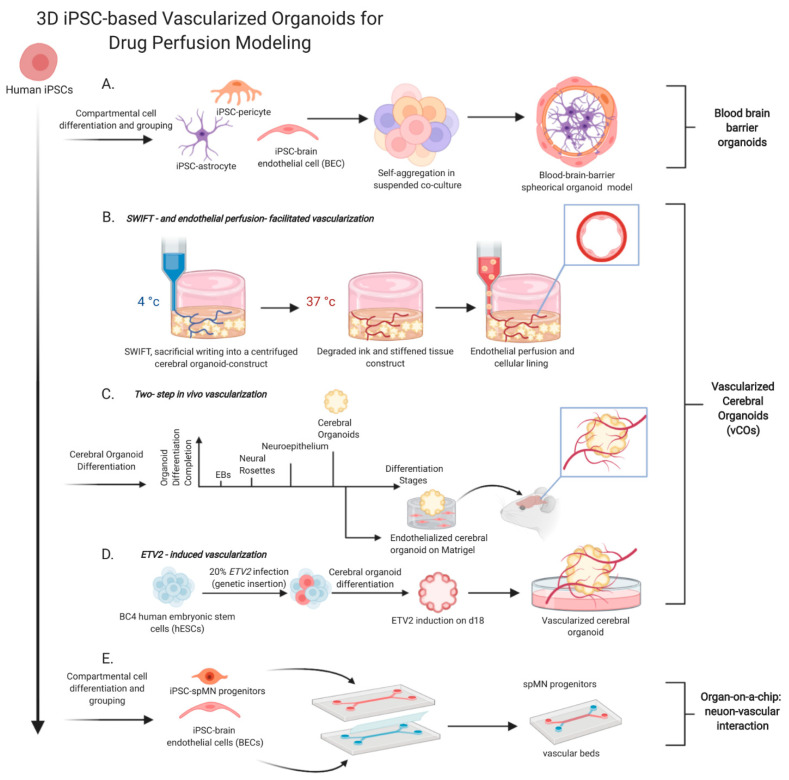
Figure 1.
3D induced pluripotent stem cell (iPSC)-based vascularized organoids for drug perfusion modeling. (A) iPSC-derived blood-brain-barrier model. Astrocytes, pericytes and endothelial cells are generated from human iPSCs and mixed in suspension for self-aggregation. The resulted spheroid comprises an inner layer of astrocytes, intermediate layer of pericytes, and an outer layer of endothelial cells. (B) Scaffold-dependent vascularization of organoids through sacrificial writing into functional tissues (SWIFT). Cerebral organoids are mixed and compacted into a solid-like construct in an appropriate extracellular matrix medium. The sacrificial gelatin ink is 3D typed into the tissue construct. The tissue construct solidifies with increased temperature, while the sacrificial ink melts away, leaving tubular structures embedded within. Perfusion of endothelial cells endothelializes the interior of the channels and form vascular beds. (C) External revascularization of human iPSC-cerebral organoids within mouse brain cavity. Non-vascularized organoids are seeded onto endothelial containing matrigel droplets DIV 34 and transplanted onto raw edges of the resection cavity in the mouse brain. (D) ETV2-facilitated endogenous vascularization of cerebral organoids in vitro. A mixture of BC4 hECs and ETV2-infected BC4 hECs is prepared with a 20% cell infection rate. On day 18 of organoid differentiation, viral induction is initiated. Endothelial tubes are formed along the organoid differentiation and continuously evolve with increased structural complexity. (E) A dual-channeled microifluidic organ/organ-on-a chip (OoC) to model neural-vascular interactions. Human iPSC-derived spinal neural progenitors (hiPSC-spNPs) are seeded on the top channel and iPSC-derived brain endothelial cells (BECs) on the bottom. Vasculature develops as the neural progenitors differentiate into spinal neurons with mediated signaling between two different cell types.