Abstract
Simple Summary
Intrahepatic cholangiocarcinoma (IHCC) is the second most common primary hepatic malignant tumor after hepatocellular carcinoma (HCC). The prevalence of lymph node metastases (LNM) detected at surgery for IHCC has been reported as 25–50%, and lymph node metastasis is known to be significantly associated with poor survival outcomes. However, the oncologic value of lymph node dissection in resected IHCC is still controversial. According to the present Korea–Japan international collaborative study, it was found that surgical retrieval of more than four lymph nodes (≥4 LNs) could improve survival outcome in resected IHCC with LNM. Based on preoperatively detectable parameters, a nomogram was established to predict LNM to suggest tailored intraoperative LN management in patients with IHCC. Further prospective research is needed to validate the present surgical strategy in resected IHCC.
Abstract
Background: This study was performed to investigate the oncologic role of lymph node (LN) management and to propose a surgical strategy for treating intrahepatic cholangiocarcinoma (IHCC). Methods: The medical records of patients with resected IHCC were retrospectively reviewed from multiple institutions in Korea and Japan. Short-term and long-term oncologic outcomes were analyzed according to lymph node metastasis (LNM). A nomogram to predict LNM in treating IHCC was established to propose a surgical strategy for managing IHCC. Results: A total of 1138 patients were enrolled. Of these, 413 patients underwent LN management and 725 did not. A total of 293 patients were found to have LNM. The No. 12 lymph node (36%) was the most frequent metastatic node, and the No. 8 lymph node (21%) was the second most common. LNM showed adverse long-term oncologic impact in patients with resected IHCC (14 months, 95% CI (11.4–16.6) vs. 74 months, 95% CI (57.2–90.8), p < 0.001), and the number of LNM (0, 1–3, 4≤) was also significantly related to negative oncologic impacts in patients with resected IHCC (74 months, 95% CI (57.2–90.8) vs. 19 months, 95% CI (14.4–23.6) vs. 11 months, 95% CI (8.1–13.8)), p < 0.001). Surgical retrieval of more than four (≥4) LNs could improve the survival outcome in resected IHCC with LNM (13 months, 95% CI (10.4–15.6)) vs. 30 months, 95% CI (13.1–46.9), p = 0.045). Based on preoperatively detectable parameters, a nomogram was established to predict LNM according to the tumor location. The AUC was 0.748 (95% CI: 0.706–0.788), and the Hosmer and Lemeshow goodness of fit test showed p = 0.4904. Conclusion: Case-specific surgical retrieval of more than four LNs is required in patients highly suspected to have LNM, based on a preoperative detectable parameter-based nomogram. Further prospective research is needed to validate the present surgical strategy in resected IHCC.
Keywords: cholangiocarcinoma, lymph nodes, metastasis, nomograms
1. Introduction
Intrahepatic cholangiocarcinoma (IHCC) is the second most common primary hepatic malignant tumor after hepatocellular carcinoma (HCC) [1,2]. IHCC represents about 10–20% of all primary liver cancers [3]. Recently, the incidence of IHCC has also been reported to be increasing worldwide [4].
Surgical resection is the mainstay of curative-intent treatment for IHCC and is associated with improved survival in selected patients. In 1997, the Liver Cancer Study Group of Japan proposed that regional lymph nodes of IHCC should be categorized into three groups based on lymph node mapping studies [5]. Recently, the 8th American Joint Committee on Cancer (AJCC) Cancer Staging Manual also suggested that left-sided IHCC should include inferior phrenic, hilar, and gastrohepatic lymph nodes as regional lymph node groups. For right-sided IHCC, regional lymph nodes include hilar, periduodenal, and peripancreatic lymph node areas [6]. These regional lymph nodes are theoretically surgical targets that should be dissected during curative surgical procedures.
The prevalence of lymph node metastases detected at surgery for IHCC has been reported as 25–50% [7]. The literature suggests that lymph node metastasis is significantly associated with poor survival outcomes in patients who undergo hepatic resection for IHCC [8,9]. However, the oncologic value of lymph node dissection in resected IHCC is still controversial [10,11].
Since 2015, Korea and Japan have launched the first mutual collaboration study in the field of Hepato-Biliary-Pancreatic surgery. Several important achievements have already been published, and some of them are being processed for future publications [12,13,14]. The aim of the present study is to investigate the oncologic role of lymph node management in treating IHCC, based on a large-volume study population, and to propose a potential surgical strategy for managing lymph nodes when resecting IHCC.
2. Results
2.1. General Characteristics of Patients and Primary Tumors
A total of 828 and 310 patients with resected IHCC were enrolled in Korea and Japan, respectively. There were 758 male patients (66.6%) with an average age of 63.4 years. Five hundred and fifty-eight patients (50.4%) had right-sided tumors. Left hemihepatectomy was the most common procedure performed in 325 patients (28.6%, Table 1).
Table 1.
Demographics of patients with resected intrahepatic cholangiocarcinoma (IHCC).
| Patients (N = 1138) | |
|---|---|
| Sex | |
| Male | 758 (66.6%) |
| Female | 380 (33.4%) |
| Age (years) | 63.4 ± 9.8 |
| Previous symptoms | |
| Yes | 535 (47.0%) |
| No | 603 (53.0%) |
| American Sosciety of Anesthesiologists (ASA) score | |
| 1 | 250 (24.6%) |
| 2 | 662 (65.2%) |
| 3 | 98 (9.6%) |
| 4 | 1 (0.1%) |
| 5 | 1 (0.1%) |
| Karnofsky scale | |
| 50 | 1 (0.1%) |
| 60 | 5 (0.5%) |
| 70 | 25 (2.6%) |
| 80 | 142 (14.8%) |
| 90 | 518 (54.1%) |
| 100 | 267 (27.9%) |
| Tumor location side | |
| Right liver | 558 (50.4%) |
| Left liver | 549 (49.6%) |
| Operation name | |
| Lt. lateral sectionectomy | 75 (6.6%) |
| Lt hemihepatectomy | 325 (28.6%) |
| Lt extended hepatectomy | 126 (11.1%) |
| Rt hemihepatectomy | 295 (25.9%) |
| Rt extended hepatectomy | 79 (6.9%) |
| Trisectionectomy | 31 (2.7%) |
| Bisegmentectomy | 65 (5.7%) |
| Segmentectomy | 142 (12.5%) |
| Lymph node retrieval | |
| No | 413 (36.3%) |
| Yes | 725 (63.7%) |
| Serum Carcinoembryonic antigen (CEA) (ng/mL) | 21.5 ± 111.3 |
| Serum 19-9 (U/mL) | 2273.9 ± 10,816.4 |
Most patients underwent combined caudate lobectomy (934 patients, 82.4%). Seven hundred and twenty-five patients (63.7%) underwent surgical management for lymph node retrieval. Combination resection of the diaphragm was the most common procedure, excluding the gallbladder (Table S1).
Mean pathologic tumor size was 5.1 ± 3.0 cm, and the mean number of retrieved lymph nodes was six (5.9 ± 8.3). The mean number of metastatic lymph nodes was one (1.1 ± 2.5). The R0 resection rate was 89% with a tumor safety margin of 10.9 ± 14.3 mm (Table S2).
Median disease-free survival was 18 months (95% CI (14.93–21.07)), and median overall disease-specific survival was 40 months (95% (33.54–46.46)) in patients with resected IHCC.
2.2. Distribution of Metastatic Lymph Nodes in Patients with Resected IHCC
Seven-hundred twenty-five patients with resected IHCC (63.7%) were noted to have retrieved lymph nodes. The number of retrieved lymph nodes was 5.9 ± 8.3. Among them, lymph node metastasis was observed in 293 patients (N1, 25.7%), and 432 patients (N0, 38.0%) were found to have no lymph node metastasis. Lymph node status could not be assessed in 413 patients (NX, 36.3%).
Analysis of topographical distribution of metastatic lymph nodes showed that the No. 12 lymph node (36%) was the most frequent metastatic node, and the No. 8 lymph node (21%) was the second most common metastatic node. (Figure S1).
In addition, left-sided IHCC was significantly associated with lymph node metastasis (right-sided, 112 of 338 patients (33.1%) vs. left-sided, 181 of 387 patients (46.8%), p < 0.001). Right-sided IHCC was related to the No. 12 lymph nodes (p = 0.065) and the No. 13 lymph nodes (p = 0.024, Table 2) (Table S3).
Table 2.
Topographical relationship between metastatic lymph nodes and primary tumor location of resected IHCC.
| Tumor Side | Right-Sided (N = 112) |
Left-Sided (N = 181) |
p |
|---|---|---|---|
| No. 7 Lymph node (LN) | 0.013 | ||
| No | 109 (97.3%) | 162 (89.5%) | |
| Metastasis | 3 (2.7%) | 19 (10.5%) | |
| No. 12 LN | 0.065 | ||
| No | 47 (42.0%) | 96 (53.0%) | |
| Metastasis | 65 (58.0%) | 85 (47.0%) | |
| No. 13 LN | 0.024 | ||
| No | 93 (83.0%) | 166 (91.7%) | |
| Metastasis | 19 (17.0%) | 15 (8.3%) | |
| Perigastric LN | 0.052 | ||
| No | 109 (97.3%) | 166 (91.7%) | |
| Metastasis | 3 (2.7%) | 15 (8.3%) |
2.3. Oncologic Impact of Metastatic Lymph Nodes in Patients with Resected IHCC
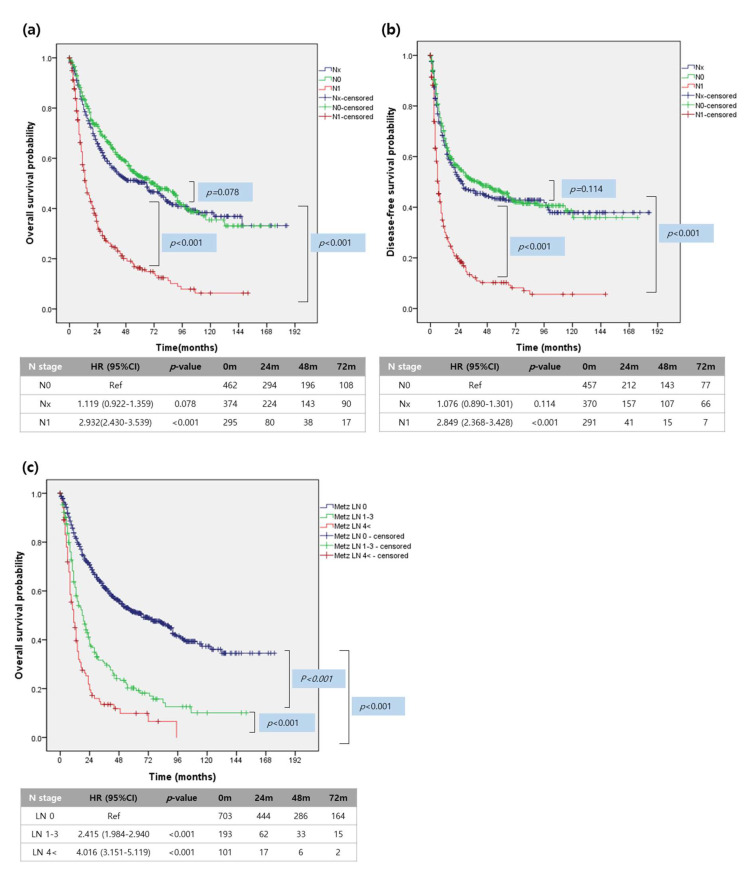
Metastatic lymph nodes showed adverse long-term oncologic impact in patients with resected IHCC. N1 patients (median 14 months, 95% CI (11.4–16.6)) showed worse disease-specific survival compared with NX patients (median 58 months, 95% CI (40.2–75.8), p < 0.001) and N0 patients (median 74 months, 95% CI (57.2–90.8), p < 0.001, Figure 1a).
Figure 1.
Survival analysis of resected IHCC according to N-stage. (a) Overall survival survival of resected IHCC (b) Disease-free survival analysis. (c) Overall survival according to the number of metastatic lymph nodes.
A similar pattern of survival was also observed in the disease-free survival analysis. N1 patients (median disease-free survival 6 months, 95% CI (4.9–7.0)) showed inferior survival outcomes than the other two groups (Nx patients: median disease-free survival 25 months, 95% CI (15.6–34.4) and N0 patients: median disease-free survival 44 months, 95% CI (25.3–62.7), Figure 1b).
Interestingly, the number of metastatic lymph nodes was also significantly related to a negative oncologic impact in patients with resected IHCC. Patients with ≥4 metastatic lymph nodes (median 11 months, 95% CI (8.1–13.8)) showed the worst long-term oncologic outcome compared to those with one to three metastatic lymph nodes (median 19 months, 95% CI (14.4–23.6), p < 0.001) and zero metastatic lymph nodes (median 74 months, 95% CI (57.2–90.8), p < 0.001, Figure 1c).
2.4. Oncologic Impact of Number of Retrieved Lymph Nodes on N0, and N1 Patients with Resected IHCC
Among all patients with known lymph node status (N = 725 patients), the number of retrieved lymph nodes was not associated with significant survival differences in patients with resected IHCC (Figure S2).
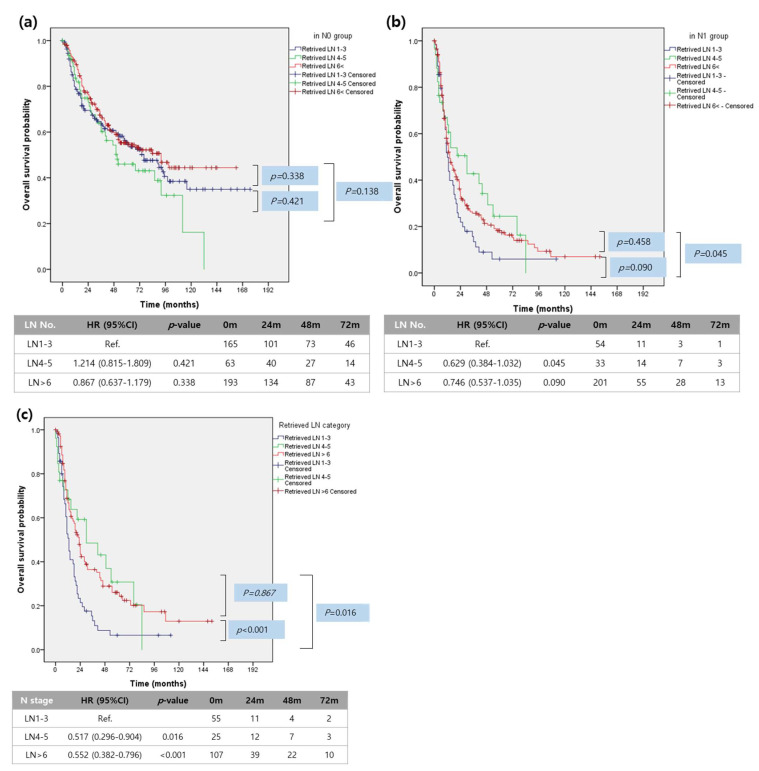
However, the significant oncologic impact of the number of retrieved lymph nodes was observed according to lymph node metastasis status in patients with resected IHCC. In N0 patients, the number of retrieved lymph nodes was not associated with long-term survival differences (Figure 2a). However, in N1 patients, the number of retrieved lymph nodes was significantly associated with survival differences. Patients with one to three retrieved lymph nodes (median 13 months, 95% CI (10.4–15.6)) had poor survival outcomes compared to those with four to five retrieved lymph nodes (median 30 months, 95% CI (13.1–46.9), p = 0.045) and ≥six retrieved lymph nodes (median 14 months, 95% CI (10.9–17.1), p = 0.090). There were no statistically significant survival differences between patients with four to five retrieved lymph nodes and those with ≥six retrieved lymph nodes (p = 0.458, Figure 2b), suggesting that surgical effort to retrieve more than four lymph nodes will benefit patients with lymph node metastasis.
Figure 2.
Survival analysis according to number of retrieved lymph nodes. (a) Overall survival analysis according to the number of retrieved lymph nodes in the N0 group. (b) Overall survival analysis according to the number of retrieved lymph nodes in the N1 group. (c) Overall survival analysis according to the number of retrieved lymph nodes in patients with less than 4 (1–3) metastatic lymph nodes.
Analysis of the oncologic impact of the number of retrieved lymph nodes according to the number of metastatic lymph nodes clearly demonstrates that any effort to retrieve additional lymph nodes (four to five, vs. six<) does not provide a positive effect on the long-term survival of patients with more than four metastatic lymph nodes (median survival 9 months (95% CI: 0.0001–20.087) vs. median survival 11 months (95% CI: 8.283–13.717), p = 0.989), However, surgical effort to remove more than four (four to five, and six < vs. one to three) lymph nodes resulted in an improved long-term survival outcome in patients with less than four metastatic lymph nodes (median survival 30 months, (95% CI: 3.984–56.016), p = 0.016, and median survival 23 months, 95% (CI: 18.775–27.225), p = 0.001, vs. median survival 13 months (95% CI: 10.4–15.6), Figure 2c), suggesting that surgical retrieval of lymph nodes plays a significant oncologic role in some patients with limited lymph node metastasis.
2.5. Can Lymph Node Metastasis Be Preoperatively Predicted in Patients with Resected IHCC? Developing a Surgeon-Oriented Nomogram to Predict Lymph Node Metastasis
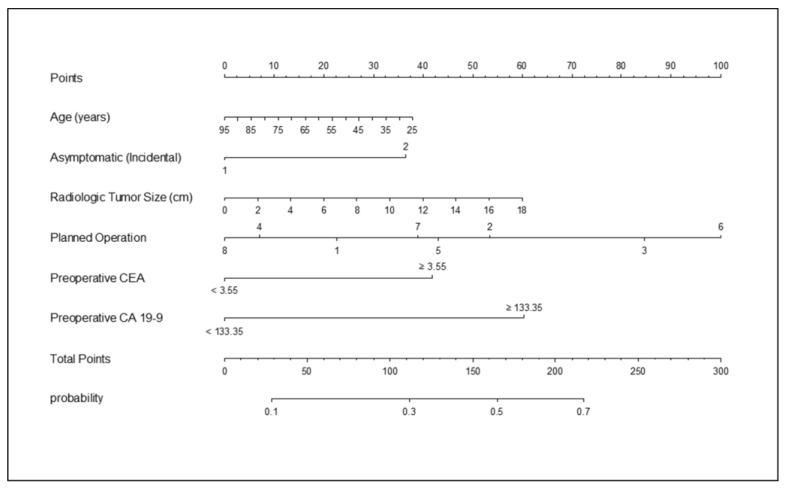
Among the detectable preoperative parameters that are thought be clinically available in usual clinical practice, age, symptoms, radiologic tumor size, planned operation, and preoperative tumor markers were considered to establish a statistical model to preoperatively predict lymph node metastasis in patients with IHCC (Table 3 and Figure 3). Logistic regression analysis identified symptoms at diagnosis (OR = 1.803 (95% CI: 1.245–2.612), p = 0.0018), operation type (for example, left extended hemihepatectomy, OR = 2.713 (95% CI 1.079–6.825), p = 0.0339), preoperative serum CEA level (OR = 1.966 (95% CI: 1.352–2.857), p = 0.0004), and preoperative serum CA 19-9 level (OR = 2.648 (95% CI: 1.837–3.819), p < 0.001) as independent predictors for lymph node metastasis.
Table 3.
Univariate and multivariate analysis for preoperatively predicting lymph node metastasis in resected IHCC.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Sex (Male/Female) | 1.08 (0.798–1.463) | 0.6164 | ||
| Age, years | 0.989 (0.974–1.004) | 0.1401 | 0.991 (0.974–1.009) | 0.3427 |
| Chief complaint (no/yes) | 1.81 (1.344–2.438) | 0.0001 | 1.803 (1.245–2.612) | 0.0018 |
| ASA | 0.727 (0.505–1.046) | 0.0854 | ||
| 0.873 (0.479–1.591) | 0.6575 | |||
| Karnofsky score | 0.995 (0.975–1.016) | 0.657 | ||
| Radiologic tumor size, cm | 1.092 (1.033–1.156) | 0.0021 | 1.055 (0.982–1.134) | 0.1415 |
| Gross type | 0.917 (0.748–1.124) | 0.4023 | ||
| Tumor location (Right/Left) | 1.546 (1.148–2.082) | 0.0042 | ||
| Number of the tumor | 1.303 (0.985–1.723) | 0.064 | ||
| Left hemihepatectomy | 1.122 (0.555–2.268) | 0.7495 | 1.646 (0.685–3.952) | 0.2649 |
| Left extended hemihepatectomy | 1.608 (0.755–3.425) | 0.2185 | 2.713 (1.079–6.825) | 0.0339 |
| Right hemihepatectomy | 0.651 (0.315–1.343) | 0.2448 | 0.777 (0.319–1.896) | 0.5799 |
| Right extended hemihepatectomy | 1.004 (0.44–2.288) | 0.9934 | 1.39 (0.515–3.752) | 0.5156 |
| Trisectionentectomy | 2.514 (0.804–7.862) | 0.1129 | 3.488 (0.925–13.15) | 0.0651 |
| Bisegmentectomy | 0.933 (0.365–2.384) | 0.8854 | 1.301 (0.411–4.117) | 0.6539 |
| Segmentectomy | 0.304 (0.121–0.766) | 0.0116 | 0.694 (0.233–2.066) | 0.5119 |
| Preoperative CEA | 2.113 (1.537–2.903) | <0.0001 | 1.966 (1.352–2.857) | 0.0004 |
| Preoperative CA 19-9 | 3.389 (2.475–4.643) | <0.0001 | 2.648 (1.837–3.819) | <0.0001 |
Figure 3.
Nomogram developed to preoperatively predict lymph node metastasis in patients with IHCC. Presence/absence of symptoms, one = no symptoms, two = symptomatic; planned operation, one = left lateral segmentectomy, two = left hemihepatectomy, three = left extended hemihepatectomy, four = right hemihepatectomy, five = right extended hemihepatectomy, six = trisectionectomy, seven = bisegmentectomy, eight = segmentectomy.
The nomogram AUC was 0.737, and internal validation using 1000 bootstrap sampling from the primary cohort showed an AUC of 0.748 (95% CI: 0.706–0.788). These values suggest a stable accurate capacity for preoperatively predicting lymph node metastasis. In addition, the apparent performance of the nomogram to preoperatively predict lymph node status was tested in N0 and N1 patients. The calibration curve of the nomogram for the probability of LN metastasis demonstrated a good level of agreement between prediction and observation in the primary cohort (Hosmer and Lemeshow goodness of fit test, p = 0.4904, Figure S3).
2.6. Indirect External Validation of Nomogram in Nx Patients with Resected IHCC
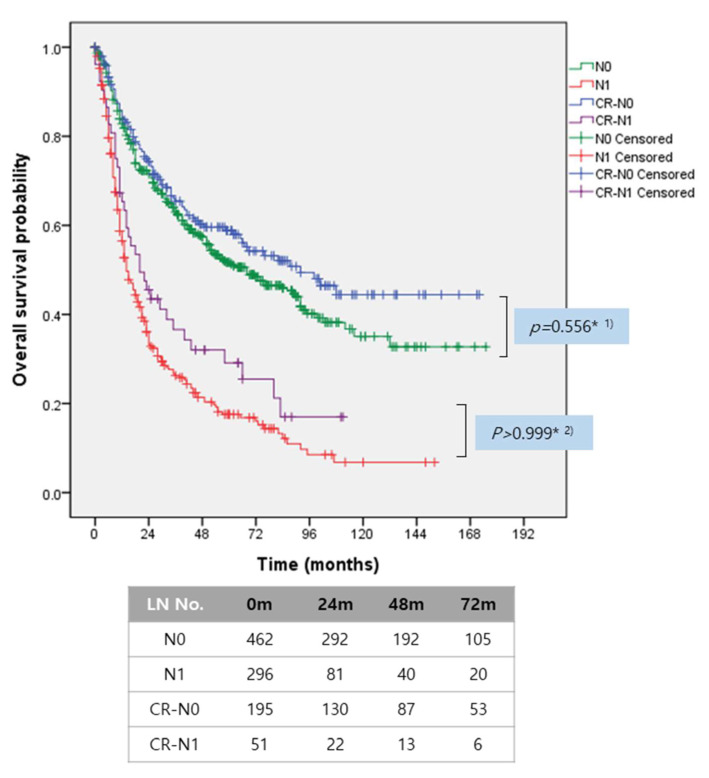
The developed nomogram was applied in Nx patients with resected IHCC, whose lymph node status was not assessed, to estimate the discriminating capacity for predicting lymph node metastasis in patients with resected IHCC. Nx patients were categorized into two groups according to the calculated probability of lymph node metastasis. There were significant survival differences between Nx patients with low risk of lymph node metastasis (<50%) and those with high risk of lymph node metastasis (≥50%, p = 0.0002). When superimposing known survival outcomes of patients with resected IHCC according to lymph node metastasis, the survival outcomes of Nx patients with low risk of lymph node metastasis were similar to actual N0 patients (p = 0.0793, Bonferroni-corrected p-value = 0.5558). The survival outcomes of Nx patients with high risk of lymph node metastasis also showed no significant difference from that of known N1 patients (p = 0.5610, and Bonferroni-corrected [15] p-value > 0.99999, Figure 4). Therefore, in an effort to improve survival outcomes, Nx patients with high risk of lymph node metastasis should have undergone surgical management to retrieve at least four lymph nodes during hepatectomy.
Figure 4.
Correlation of survival outcomes between actual lymph node status and calculated risk of lymph node metastasis in resected IHCC. Survival outcomes according to calculated risk of lymph node metastasis were similar to those according to actual lymph node metastasis, (1) Bonferroni corrected p-value = 0.5558, (2) Bonferroni-corrected p-value > 0.9999, CR, calculated risk.
2.7. Proposed Surgical Strategy in Lymph Node Management for IHCC
The following surgical strategy is proposed for managing lymph nodes during resection of IHCC. According to the calculated risk of lymph node metastasis based on the present surgeon-oriented nomogram, web-based and case-specific lymph node management can be suggested for surgical candidates with IHCC (http://40.121.207.11:8080/home3.jsp). This model calculates not only the risk of lymph node metastasis, but also provides a potential area of lymph node metastasis. In patients with a high calculated risk of lymph node metastasis (≥50%), surgical retrieval of at least four lymph nodes is recommended. Removal of different regional lymph nodes according to primary tumor location was considered, including the porta hepatis area (the most common location of metastatic lymph nodes), the No. 8 LNs (second most common location of metastatic lymph nodes), and the No. 7/perigastric LNs (common in left-sided IHCC). Conversely, patients with a low calculated risk of lymph node metastasis (<50%) only require lymph node sampling around the portahepatis for accurate tumor staging. In clinical practice, specimens can be sent for pathological evaluation as frozen-section biopsies for further decision making (Figure S4).
3. Discussion
The present Korean–Japan collaborative study to investigate the oncologic role of surgical effort to remove lymph nodes demonstrated that lymph node metastasis was strongly associated with poor long-term oncologic outcomes in resected IHCC. The present study emphasizes that not only lymph node metastasis status but also the number of metastatic lymph nodes should be considered when stratifying patients with resected IHCC and lymph node metastasis. Several validation studies are currently available that support the notion that the number of metastatic lymph nodes is more reliable and accurate for estimating long-term oncologic outcomes in resected pancreatic cancer [16,17,18,19] and gallbladder cancer [20,21].
Moreover, surgical retrieval of lymph nodes can have varying degrees of oncologic significance, according to the status of lymph node metastasis when managing IHCC. This observation is thought to be an important result of our study. There was no significant oncologic impact of the number of retrieved lymph nodes in resected IHCC without lymph node metastasis (N0). However, the number of retrieved lymph nodes provided a positive impact on the long-term survival in resected IHCC with lymph node metastasis (N1). The present study suggests that surgical effort to remove at least four lymph nodes is required in resection of IHCC with N1 to significantly impact long-term oncologic outcomes.
The long-term oncologic outcomes of patients with IHCC and lymph node metastasis are influenced not only by tumor biology, but also by surgical effort to remove regional lymph nodes. Any surgical effort to remove lymph nodes was not associated with improved survival outcome. Conversely, those with a limited number of metastatic lymph nodes (one to three) clearly demonstrated that surgical retrieval of at least four lymph nodes could provide a positive oncologic impact in resected IHCC. This observation may explain the controversial data on the issue of lymph node management in resected IHCC [22,23,24,25,26,27]. Studies enrolling many patients with ≥four metastatic lymph nodes may conclude that lymph node dissection does not play a significant role in managing IHCC.
In this study, a nomogram was developed to predict lymph node metastasis in resected IHCC based on preoperative detectable parameters because surgical management of lymph nodes can differ according to the presence of lymph node metastasis. Several studies have proposed nomograms for preoperatively predicting lymph node metastasis in resected IHCC. Meng et al. [28] proposed a nomogram based on preoperative serum CA 19-9, primary tumor site, measured lymph node size based on a CT scan, and tumor growth pattern. Huwan et al. [29] also proposed a radiomics nomogram for the preoperative prediction of lymph node metastasis.
It is more practical and applicable if a surgeon-oriented nomogram to preoperatively predict lymph node metastasis is proposed. The present nomogram is web-based operating system. Therefore, it can be used in every clinical office and even in operative theaters to provide additional information for appropriate decision-making regarding lymph node management for IHCC. Our proposed nomogram is simple and practical. Surgeons can apply the present nomogram to provide patients and their families with explanations on probable lymph node status even in the preoperative setting. To calculate the risk of lymph node metastasis, surgeons only need to know patient age, symptoms (incidental finding or symptomatic), radiologic tumor size (cm), the planned surgical extent of hepatectomy, preoperative serum CEA, and preoperative CA 19-9. This information can be obtained while interviewing patients in the preoperative setting and even during operation. Internal validation showed that this nomogram has an acceptable level of accuracy and predictive capacity to estimate lymph node metastasis status (AUC, 0.748, 95% CI: 0.704–0.788, and Hosmer and Lemeshow goodness of fit test, p = 0.4904), similar to two previous reports [28,29]. When the present nomogram was applied in 413 patients whose lymph node status was not assessed (Nx patients), patients were categorized into two groups with discrete long-term survival outcome according to the calculated risk of lymph node metastasis. In turn, the long-term oncologic outcomes of Nx patients were exactly superimposed on those of patients with known lymph node metastasis. Our study emphasizes that Nx patients with a high calculated risk of lymph node metastasis (≥50%) could obtain oncologic benefit from surgical retrieval of at least four lymph nodes during curative resection.
This study is based on the largest study populations of resected IHCC. However, there are several limitations. First, the study design is retrospective, so it is difficult to identify the exact location of retrieved LNs, the exact extent of LN dissection, and the cN-stage to improve the accuracy for predicting metastatic LNs. According to the present review of topographic location of metastatic lymph nodes, the No. 12 and No. 8 lymph nodes were the most frequent occurring nodes. It is well known that regional lymph nodes differ according to the location of the primary IHCC [30]. Systemic lymph node dissection according to tumor location, including the No. 12 and No. 8 areas, is suggested to obtain more than four lymph nodes when lymph node metastasis is highly suspected by nomogram or by intraoperative frozen section biopsy of suspicious enlarged lymph nodes. Secondly, a fairly large number of patients (N = 413, 36.3%) were noted to have Nx (the amount of harvested lymph nodes and amount of positive lymph nodes could not be assessed), which could potentially introduce bias in the study. This may be a result of different surgical interventions towards LNs (surgeon bias), and non-standardized pathologic evaluation methods (pathologist bias). In this study, we did not exclude Nx patients because this group was used for only indirect validation. In addition, Nx is categorized as one of the N staging systems (Nx, N0, and N1), and Nx can definitely occur in our clinical practice. However, for further in depth analysis of the oncologic role of LNM in resected IHCC, a well designed prospective study will be necessary. Lastly, the accuracy of the present nomogram was tested by only internal validation. Instead, the potential capacity of the proposed nomogram to predict lymph node metastasis was indirectly shown in Nx patients with resected IHCC, not by external validation using a different cohort of patients. Therefore, a well-designed external validation study based on prospective study protocol should be performed in the near future to strengthen the value of the proposed nomogram-based case-specific LN management strategy in resected IHCC.
4. Materials and Methods
Study design and data collection: This study was approved by the Korea Association of Hepato-Biliary-Pancreatic Surgery and the Japanese Society of Hepato-Biliary-Pancreatic Surgery as an international multicenter collaborative study. Patients with resected IHCC were selected from digitalized patient records of individual institutions who agreed with the present international collaborative study protocol. From January 2000 to December 2011, the medical records of patients who underwent potential surgical resection for IHCC were retrospectively reviewed. The clinical and pathological parameters, including the number of retrieved lymph nodes, metastatic lymph nodes, and long-term survival data, were collected from medical records of individual patients to establish the present data-base for the Korea–Japan collaborative study. This study was approved by the Institutional Review Board of Yonsei University College of Medicine (IRB# 4-2014-0850).
Statistics: All data were collected and analyzed at the Department of Surgery and Department of Biostatistics, Yonsei University College of Medicine, Seoul, Korea. Continuous variables were described as mean ± standard deviation, and categorical variables were described as frequency (%). Student’s t-test, chi-square test, or Fisher’s exact test were performed. Survival was assessed with Kaplan–Meier analyses. Survival outcomes were compared using log–rank tests with 95% confidence interval (CIs) to identify associations between clinical factors and survival outcomes.
When developing the prediction model, the Youden Index [31] was used to determine the optimal cutoff points for preoperative serum CA19-9 and CEA. Preoperatively available clinical parameters that achieved statistical significance in the univariable logistic regression model and were considered of clinical importance were included in the final model. A nomogram was developed by using the package rms in R version 3.1.3 (http://www.r-project.org/) on the basis of the results of the multivariable logistic regression model. Internal validation was performed using 1000 bootstrapped resamples, which were also used to generate the calibration plot. To quantify the discriminative ability of the final model, AUC was measured. Calibration was evaluated by calibration plot, a graphic representation of the relationship between observed and predicted probability. The Hosmer–Lemeshow goodness-of-fit test was performed [32]. The agreement between predicted and observed probability was assessed, and p-value > 0.05 indicates good calibration. To further assess the validation, Kaplan–Meier curves of Nx patients were plotted according to calculated risk of lymph node metastasis by nomogram over risk stratified groups by actual lymph node metastasis. p-values < 0.05 were considered statistically significant. To strictly validate the oncologic significance of the calculated risk of lymph node metastasis, Bonferroni-corrected p-values were applied when comparing actual survival.
5. Conclusions
In summary, the present Korea–Japan collaborative study suggests a potential strategy to tailor surgical approaches based on the calculated risk of lymph node metastasis when treating IHCC. Patients with high risk of lymph node metastasis should be treated by lymph node dissection to obtain more than four lymph nodes during IHCC resection. Patients with a low risk of lymph node metastasis should simply be managed by margin-negative hepatectomy with lymph node sampling to avoid unnecessary lymph node management and to ensure accurate staging. This surgical concept should be approved by a multi-national and multi-center randomized control study with reasonable consensus-based study protocol. Future research on this topic is needed.
Acknowledgments
Authors have acknowledgements for Korea Association of Hepato-Biliary-Pancreatic Surgery and the Japanese Society of Hepato-Biliary-Pancreatic Surgery.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/3/445/s1, Table S1: Operative findings of patients with resected IHCC. Table S2: Pathologic findings of patients with resected IHCC. Table S3: Topographic relationship between metastatic lymph nodes and primary tumor location of resected IHCC. Figure S1: Status of metastatic and retrieved lymph nodes in resected IHCC. Figure S2: Overall impact of the number of retrieved lymph nodes in patients with resected IHCC. Figure S3: Accuracy of developed nomogram to preoperatively predict lymph node metastasis in patients with resected IHCC. Figure S4: Proposal for case-specific surgical management of lymph nodes in treating IHCC.
Author Contributions
C.M.K.: conceptualization, methodology, validation, writing—original draft, editing and visualization. K.-S.S., N.-J.Y., T.H.H., S.J.P., K.S.A., H.H., S.B.C., C.-Y.J., T.T., S.S. (Shigehiro Shiozaki), Y.H.R., H.C.Y., T.F., R.M., U.N., K.H., H.I.S., T.O., T.K., S.S. (Sohei SATOI), H.N., H.K., K.T.: methodology, data collection, validation, investigation, S.K., D.W.C.: conceptualization, methodology, validation, writing—review and editing, and supervising. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Yonsei University College of Medicine (IRB#4-2014-0850, 2014-11-25).
Informed Consent Statement
Patient consent was waived due to its retrospective nature of the study design.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to IRB restriction regarding personal information.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aljiffry M., Abdulelah A., Walsh M., Peltekian K., Alwayn I., Molinari M. Evidence-based approach to cholangiocarcinoma: A systematic review of the current literature. J. Am. Coll. Surg. 2009;208:134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Massarweh N.N., El-Serag H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24 doi: 10.1177/1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridgewater J., Galle P.R., Khan S.A., Llovet J.M., Park J.W., Patel T., Pawlik T.M., Gores G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Liver Cancer Study Group of Japan General rules for the clinical and pathological study of primary liver cancer. Jpn. J. Surg. 1997;19:98–129. doi: 10.1007/BF02471576. [DOI] [PubMed] [Google Scholar]
- 6.Amin M.B. AJCC Cancer Staging Manual. 8th ed. Springer; Chicago, IL, USA: 2016. [Google Scholar]
- 7.Uenishi T., Yamamoto T., Takemura S., Kubo S. Surgical treatment for intrahepatic cholangiocarcinoma. Clin. J. Gastroenterol. 2014;7:87–93. doi: 10.1007/s12328-014-0460-z. [DOI] [PubMed] [Google Scholar]
- 8.Amini N., Ejaz A., Spolverato G., Maithel S.K., Kim Y., Pawlik T.M. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: A systematic review. J. Gastrointest. Surg. 2014;18:2136–2148. doi: 10.1007/s11605-014-2667-1. [DOI] [PubMed] [Google Scholar]
- 9.Hyder O., Marques H., Pulitano C., Marsh J.W., Alexandrescu S., Bauer T.W., Gamblin T.C., Sotiropoulos G.C., Paul A., Barroso E., et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: An Eastern and Western experience. JAMA Surg. 2014;149:432–438. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- 10.Adachi T., Eguchi S. Lymph node dissection for intrahepatic cholangiocarcinoma: A critical review of the literature to date. J. Hepatobiliary Pancreat. Sci. 2014;21:162–168. doi: 10.1002/jhbp.30. [DOI] [PubMed] [Google Scholar]
- 11.Guro H., Kim J.W., Choi Y., Cho J.Y., Yoon Y.S., Han H.S. Multidisciplinary management of intrahepatic cholangiocarcinoma: Current approaches. Surg. Oncol. 2017;26:146–152. doi: 10.1016/j.suronc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Jang J.Y., Park T., Lee S., Kim Y., Lee S.Y., Kim S.W., Kim S.C., Song K.B., Yamamoto M., Hatori T., et al. Proposed Nomogram Predicting the Individual Risk of Malignancy in the Patients with Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2017;266:1062–1068. doi: 10.1097/SLA.0000000000001985. [DOI] [PubMed] [Google Scholar]
- 13.Kim D.S., Kim B.W., Hatano E., Hwang S., Hasegawa K., Kudo A., Ariizumi S., Kaibori M., Fukumoto T., Baba H., et al. Surgical Outcomes of Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Korea-Japan Multicenter Study. Ann. Surg. 2018 doi: 10.1097/SLA.0000000000003014. [DOI] [PubMed] [Google Scholar]
- 14.Kaibori M., Kon M., Kitawaki T., Kawaura T., Hasegawa K., Kokudo N., Ariizumi S., Beppu T., Ishizu H., Kubo S., et al. Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J. Hepatobiliary Pancreat. Sci. 2017;24:616–626. doi: 10.1002/jhbp.502. [DOI] [PubMed] [Google Scholar]
- 15.Dunnett C.W. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. J. Am. Stat. Assoc. 1955;50:1096–1121. doi: 10.1080/01621459.1955.10501294. [DOI] [Google Scholar]
- 16.Kimura K., Amano R., Nakata B., Yamazoe S., Hirata K., Murata A., Miura K., Nishio K., Hirakawa T., Ohira M., et al. Clinical and pathological features of five-year survivors after pancreatectomy for pancreatic adenocarcinoma. World J. Surg. Oncol. 2014;12:360. doi: 10.1186/1477-7819-12-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picozzi V.J., Oh S.Y., Edwards A., Mandelson M.T., Dorer R., Rocha F.G., Alseidi A., Biehl T., Traverso L.W., Helton W.S., et al. Five-Year Actual Overall Survival in Resected Pancreatic Cancer: A Contemporary Single-Institution Experience from a Multidisciplinary Perspective. Ann. Surg. Oncol. 2017;24:1722–1730. doi: 10.1245/s10434-016-5716-z. [DOI] [PubMed] [Google Scholar]
- 18.Winter J.M., Cameron J.L., Campbell K.A., Arnold M.A., Chang D.C., Coleman J., Hodgin M.B., Sauter P.K., Hruban R.H., Riall T.S., et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Ferrone C.R., Pieretti-Vanmarcke R., Bloom J.P., Zheng H., Szymonifka J., Wargo J.A., Thayer S.P., Lauwers G.Y., Deshpande V., Mino-Kenudson M., et al. Pancreatic ductal adenocarcinoma: Long-term survival does not equal cure. Surgery. 2012;152:S43–S49. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birnbaum D.J., Vigano L., Russolillo N., Langella S., Ferrero A., Capussotti L. Lymph node metastases in patients undergoing surgery for a gallbladder cancer. Extension of the lymph node dissection and prognostic value of the lymph node ratio. Ann. Surg. Oncol. 2015;22:811–818. doi: 10.1245/s10434-014-4044-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu G.J., Li X.H., Chen Y.X., Sun H.D., Zhao G.M., Hu S.Y. Radical lymph node dissection and assessment: Impact on gallbladder cancer prognosis. World J. Gastroenterol. 2013;19:5150–5158. doi: 10.3748/wjg.v19.i31.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guglielmi A., Ruzzenente A., Campagnaro T., Valdegamberi A., Bagante F., Bertuzzo F., Conci S., Iacono C. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J. Gastrointest. Surg. 2013;17:1917–1928. doi: 10.1007/s11605-013-2331-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X.F., Chakedis J., Bagante F., Chen Q., Beal E.W., Lv Y., Weiss M., Popescu I., Marques H.P., Aldrighetti L., et al. Trends in use of lymphadenectomy in surgery with curative intent for intrahepatic cholangiocarcinoma. Br. J. Surg. 2018;105:857–866. doi: 10.1002/bjs.10827. [DOI] [PubMed] [Google Scholar]
- 24.Lendoire J.C., Gil L., Imventarza O. Intrahepatic cholangiocarcinoma surgery: The impact of lymphadenectomy. Chin. Clin. Oncol. 2018 doi: 10.21037/cco.2018.07.02. [DOI] [PubMed] [Google Scholar]
- 25.Bagante F., Gani F., Spolverato G., Xu L., Alexandrescu S., Marques H.P., Lamelas J., Aldrighetti L., Gamblin T.C., Maithel S.K., et al. Intrahepatic Cholangiocarcinoma: Prognosis of Patients Who Did Not Undergo Lymphadenectomy. J. Am. Coll. Surg. 2015;221:1031–1040. doi: 10.1016/j.jamcollsurg.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Doussot A., Groot-Koerkamp B., Wiggers J.K., Chou J., Gonen M., DeMatteo R.P., Allen P.J., Kingham T.P., D’Angelica M.I., Jarnagin W.R. Outcomes after Resection of Intrahepatic Cholangiocarcinoma: External Validation and Comparison of Prognostic Models. J. Am. Coll. Surg. 2015;221:452–461. doi: 10.1016/j.jamcollsurg.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavros M.N., Economopoulos K.P., Alexiou V.G., Pawlik T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014;149:565–574. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 28.Meng Z.W., Lin X.Q., Zhu J.H., Han S.H., Chen Y.L. A nomogram to predict lymph node metastasis before resection in intrahepatic cholangiocarcinoma. J. Surg. Res. 2018;226:56–63. doi: 10.1016/j.jss.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Liang W., Xu L., Yang P., Zhang L., Wan D., Huang Q., Niu T., Chen F. Novel Nomogram for Preoperative Prediction of Early Recurrence in Intrahepatic Cholangiocarcinoma. Front. Oncol. 2018;8:360. doi: 10.3389/fonc.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada M., Yamashita Y., Aishima S., Shirabe K., Takenaka K., Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br. J. Surg. 2001;88:1463–1466. doi: 10.1046/j.0007-1323.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Schisterman E.F., Perkins N.J., Liu A., Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 32.Hosmer D.W., Jr., Lemeshow S., Sturdivant R.X. Applied Logistic Regression. 3rd ed. Wiley; Hoboken, NJ, USA: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to IRB restriction regarding personal information.