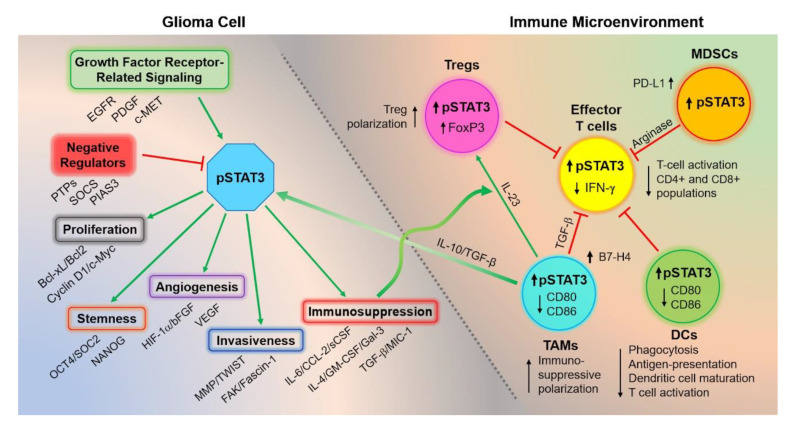
Figure 2.
STAT3 pathway activation represents a focal point of tumorigenesis and immune escape. Aberrant STAT3 activation occurs as a result of several potential upstream and downstream regulatory events including growth factor receptor signaling (e.g., epidermal growth factor (EGFR), platelet-derived growth factor (PDGF), and c-MET)), inhibition of negative regulators of STAT3 (e.g., protein tyrosine phosphatases (PTPs), suppressors of cytokine signaling (SOCS), and protein inhibitor of activated STAT 3 (PIAS3)), and microenvironmental cytokine crosstalk between immune and glioma cells. STAT3 activation transcriptionally upregulates key genes involved in proliferation, stem cell self-renewal, angiogenesis, invasiveness, and formation of the immune microenvironment. The balance of microenvironmental cytokines favors the infiltration of immunosuppressive immune cell populations, including myeloid derived suppressor cells (MDSCs), tumor-associated macrophages/microglia (TAMs), and Tregs. These cytokines activate STAT3 signaling within immune cell populations to increase immunosuppressive macrophage polarization, decreased antigen presentation, and decreased T cell activation.