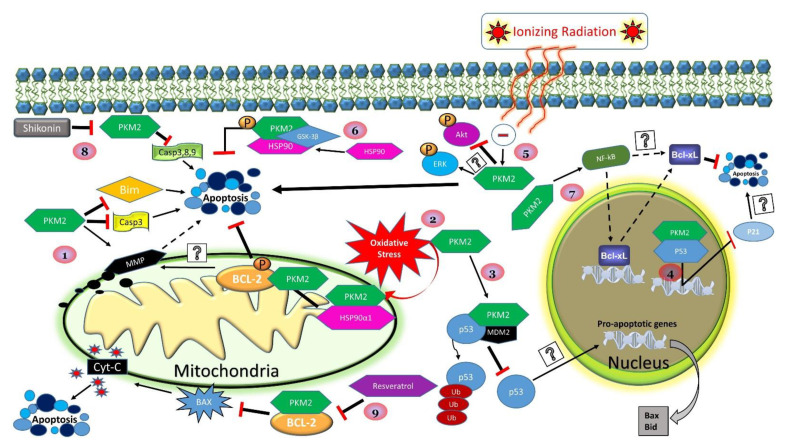
Figure 1.
Intrinsic role of PKM2 in apoptotic cancer cell death. (1) PKM2 knockdown induces apoptosis through the stabilization of Bim, decreases in mitochondrial membrane potential (MMP), and the activation of Caspase-3 [112]. (2) H2O2-induced oxidative stress promotes the mitochondrial translocation of PKM2, where it is then chaperoned by HSP90α1 in order to phosphorylate and stabilize Bcl2 [91]. (3) PKM2 forms a complex with p53 and MDM2, a master regulator protein of pro-apoptotic genes [116]. (4) In the nucleus, PKM2 interacts with P53 to reduce P53 transcriptional activity and suppress P53-induced P21 transactivation [117]. (5) Ionizing radiation-induced apoptosis is enhanced in PKM2 knockdown cells concomitant with reduced Akt phosphorylation and increased levels of phosphorylated ERK [118]. (6) HSP90 mediates the complex of PKM2 and glycogen synthase kinase-3β (GSK-3β), leading to the subsequent PKM2 phosphorylation and the inhibition of apoptosis [119]. (7) PKM2 knockdown decreases Bcl-xL gene transcription, potentially through PKM2 stabilization of NF-κB [111]. (8) Modulation of PKM2 levels and/or activity alters the cleavage/activation of caspases 3, 8, and 9 to increase apoptosis [112,120]. (9) Inhibition of PKM2 induces apoptosis through altering the Bax/Bcl-2 ratio and the subsequent release of cytochrome c from the mitochondria [121].