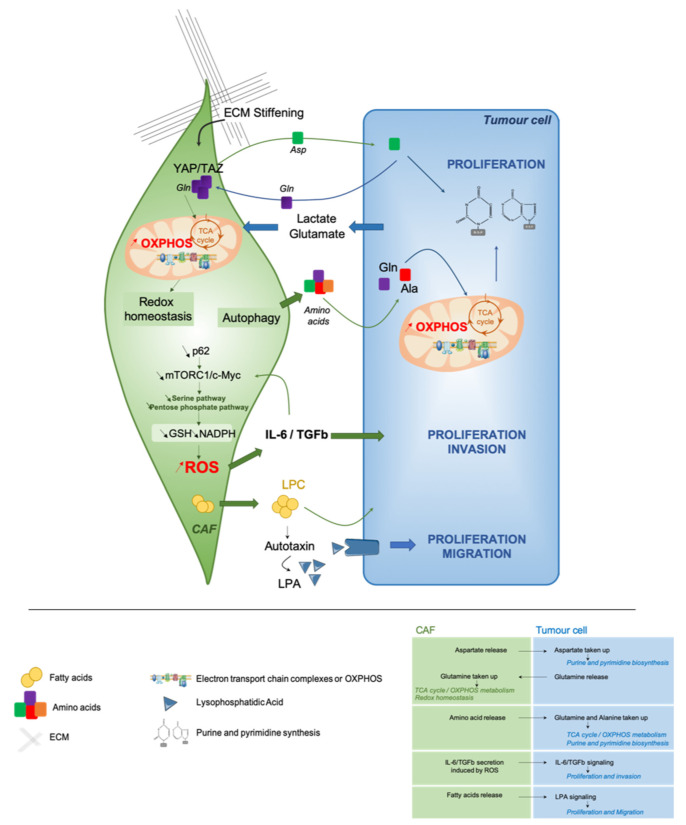
Figure 2.
Metabolic crosstalk between CAF and tumor cells. CAF have the ability to support cancer cell growth, proliferation and migration/invasion by different metabolic mechanisms involving extra cellular matrix (ECM) stiffening, redox homeostasis, autophagy and lipid secretion. ECM stiffening, through YAP/TAZ signaling promotes glutamine taken up by CAF to fuel the TCA cycle and to maintain redox homeostasis and aspartate release. Autophagy results in the release of amino acids, such as glutamine (Gln) and alanine (Ala), which can be taken up and used by cancer cells to replenish the TCA cycle and OXPHOS metabolism. Decrease mTORC1/cMYC signaling in CAF reduces serine and pentose phosphate pathways leading to a redox imbalance. Therefore, increasing reactive oxygen species (ROS) accumulation and the secretion of IL-6 and TGFβ in the microenvironment. This secretion promotes cancer cell proliferation and invasion. CAF release lipids, including lysophosphatidylcholines (LPC) into the microenvironment. LPC can be converted to lysophosphatidic acid (LPA) by extracellular autotaxin. LPA induces cancer cell proliferation and migration through the LPA receptors on cancer cells. The arrows represent the metabolites secreted or released by CAF (green) or tumor cells (blue). GSH: Reduced glutathione.