Abstract
Background
The aim of this study was to assess the survival outcomes of cisplatin-paclitaxel chemotherapy plus bevacizumab (CPB) versus cisplatin-paclitaxel chemotherapy alone (CPA) in postmenopausal women with previously untreated advanced cervical cancer (CC).
Methods
Consecutive postmenopausal women who experienced CPB or CPA were identified retrospectively from our medical centre during 2015–2019. Follow-up visits occurred 1 and 3 months after starting CPB or CPA. Afterwards, this assessment was conducted every 3 months for 1 year and then yearly thereafter. The primary endpoints were overall survival (OS) and progression-free survival (PFS); secondary endpoints were the frequency and severity of adverse events (AEs).
Results
Two hundred forty-six postmenopausal women were included (CPB, n = 124; CPA, n = 122). The median follow-up for the entire cohort was 24 months (range, 2–32). At the final follow-up, a significant difference was detected in terms of median OS (16.4 months [95% CI, 15.3–17.1] for CPB vs. 12.3 months [95% CI, 10.2–13.5] for CPA; hazard ratio (HR) 0.69, 95% CI, 0.49–0.99; p = 0.001), and the median PFS was longer in the CPB group than in the CPA group (9.2 months [95% CI, 8.3–10.7] vs. 7.9 months (95% CI, 6.1–8.6) (HR 0.62, 95% CI, 0.47–0.82; p < 0.001). There were significant differences in the number of AEs between the groups (hypertension grade ≥ 2 [p < 0.001], neutropenia grade ≥ 4 [p < 0.001], and thrombosis/embolism grade ≥ 3 [p = 0.030]).
Conclusions
Among postmenopausal women with previously untreated advanced CC, those who received CPB experienced superior survival benefits compared to those who received CPA. The safety profile for CPB was controllable despite the long duration of CPB use.
Keywords: Bevacizumab, Advanced, Cervical cancer, Chemotherapy, Survival
Background
Advanced cervical cancer (CC) continues to be an important cause of mortality among women [1–3]. The management of recurrent, persistent, or metastatic CC remains challenging, as evidenced by previous trials [1, 4, 5]. The majority of these patients receive concurrent cisplatin-based chemotherapy as the primary treatment option [6]. A newly approved regimen, the addition of bevacizumab (BEV) to combination chemotherapy, has been shown to improve survival in patients with advanced CC [1, 5]. Findings from a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) [1] indicate that cisplatin-paclitaxel plus BEV markedly improves median overall survival (OS) compared to cisplatin-paclitaxel chemotherapy alone in advanced CC (16.8 months vs. 13.3 months, respectively; hazard ratio [HR] 0.77, 95% CI, 0.62–0.95; p = 0.007). Furthermore, the survival benefit conferred by the incorporation of BEV into cisplatin-paclitaxel chemotherapy tends to be sustained with extended follow-up as evidenced by the OS curves remaining separated. However, in the phase 3 trial, it was unclear whether the selected cohort of patients had previously received chemotherapy. Moreover, these study participants were not restricted to postmenopausal women.
In 2004, BEV was approved by the United States Food and Drug Administration for specific types of cancer, and became the first antiangiogenic agent to be used in several chemotherapy regimens [1, 7, 8]. BEV is a highly purified recombinant human monoclonal IgG1 antibody, and inhibits angiogenesis by binding and neutralizing circulating vascular endothelial-derived growth factor (VEGF) [1, 7, 9]. It can inhibit tumour growth and prolong the survival of patients with advanced CC [10]. Nevertheless, the number of reports on specific groups of female patients is extremely limited [11]. Whether the previous conclusions also apply to postmenopausal women with previously untreated advanced CC remains unclear.
Herein, we performed a retrospective study to verify whether postmenopausal women with previously untreated advanced CC who received cisplatin-paclitaxel chemotherapy plus BEV (CPB) had a greater survival advantage than those who received cisplatin-paclitaxel chemotherapy alone (CPA).
Methods
Data
Consecutive postmenopausal women with previously untreated advanced CC who received CPB or CPA between January 2015 and August 2019 and for whom baseline data were available at the present analysis were identified from the First Affiliated Hospital, Sun Yat-sen University. Inclusion criteria were as follows: Chinese population; postmenopausal women with previously untreated histologically proven CC stage IV/B; advanced CC was confirmed by our institutional pathology laboratory and clinical imaging data according to FIGO 2018 cervical cancer staging criteria [12]; measurable disease; and a GOG performance status score of 0 or 1(0: fully active; 1: physically strenuous activities but ambulatory). Key exclusion criteria were as follows: prior use of targeted drug(s); previous chemotherapy, radiotherapy, chemoradiotherapy, or surgery for advanced CC; CPB or CPA discontinuation, regardless of the drug-induced adverse events (AEs); symptomatic brain metastases; cachexia; severe medical illness (such as, uncontrolled infection or hypertension, hypertension with multiple complications, HIV infection); surgical emergency (such as, intestinal obstruction); key organ function failure (such as, lung, brain, kidney, and/or heart); active bleeding conditions or coagulopathy; arterial or venous thrombosis; dementia or psychiatric disorders; and collagenosis.
Study design and treatment
A retrospective, single-centre study was conducted in which eligible postmenopausal women had underwent intravenous CPB or CPA regimen [1]. The CPB treatment consisted of cisplatin (50 mg/m2 on day 1) plus paclitaxel (175 mg/m2 on day 1) plus BEV (15 mg/kg on day 1). The CPA treatment consisted of cisplatin (50 mg/m2/day) plus paclitaxel (175 mg/m2/day). The regimens for CPB and CPA were repeated every 21 days until disease progression, death, or intolerable toxic effects. The management of hypertension in patients with advanced CC receiving BEV was consistent with previous recommendations reported by Plummer et al. [13].
Outcomes and assessments
The primary endpoints were overall survival (OS) and progression-free survival (PFS); secondary endpoints were the frequency and severity of AEs. OS was defined as the date form drug treatment to death from any cause or date last seen. PFS was defined as the date from drug treatment to the date of either disease progression or death from any cause. Postmenopausal women were defined as female patients who had not menstruated for at least a year, excluding menopause due to related diseases (i.e., endometriosis, endocrine disorders). Disease progression was defined according to the Response Evaluation Criteria in Solid Tumours (RECIST, version 1.1) [14]. Computed tomography (CT) was carried out at each follow-up. Safety was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [15]. Persistent disease was defined as not achieving a complete clinical response [8]. Pain was assessed using the Brief Pain Inventory [16]. Details related to pain and hypertension (occurrence, duration, and severity) were registered. Follow-up time was calculated from the date of clinical staging to the date last seen. Follow-up visits occurred 1 and 3 months after starting CPB or CPA. Afterwards, this assessment was conducted every 3 months for 1 year and then yearly thereafter.
Statistical analysis
Categorical variables were compared using Chi-square tests; continuous variables were compared using Student’s t-test for normally distributed variables and Mann-Whitney U test for non-normally distributed variables. Survivorship curves were completed by means of the Kaplan-Meier methods with a log-rank test. The acquisition of the hazard ratio (HR) and confidence interval (CI) were achieved through a stratified log-rank test. All statistical tests used a 2-tailed of 0.05. The survival curves were made using GraphPad Prism 8.0. To perform other statistical analysis, SPSS 26.0 (IBM, Inc., NY, America) was used.
Results
Demographic characteristics
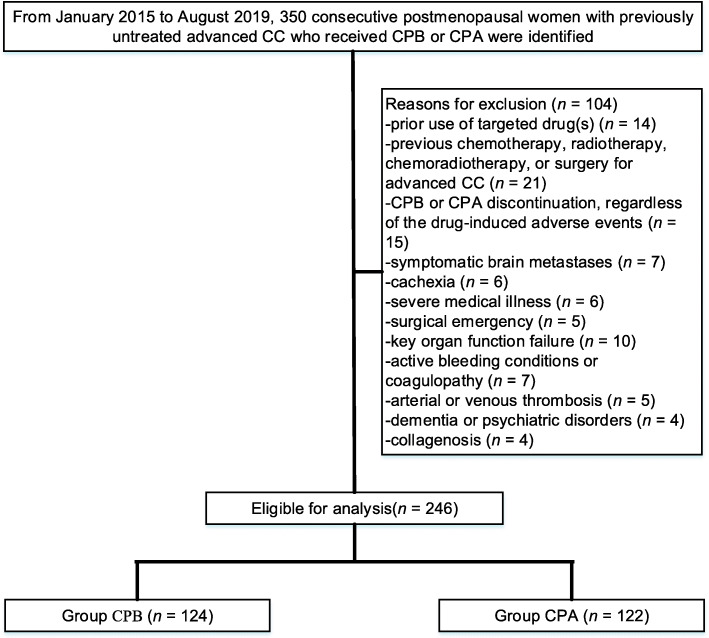
We identified 350 postmenopausal women with previously untreated advanced CC, of whom 246 postmenopausal women were included. Of these 246 women, 124 received CPB, and 122 received CPA (Fig. 1). Table 1 showed the characteristics of postmenopausal women who underwent CPB versus CPA. The mean age was 62.1[±7.59] years for CPB and 62.2[±6.84] years for CPA. ECOG performance status was 0 in 37.1% of patients and 1 in 62.9% of patients receiving CPB versus 0 in 34.4% of patients and 1 in 65.6% of patients receiving CPA (p = 0.663). Statistically significant differences were not detected with respect to age, body mass index (BMI), haemoglobin < 11 g/dL, histology, IV/B CC, duration of drug, GOG performance status, and number of metastatic sites. The median follow-up for the entire cohort was 24 months (range, 2–32). The median number of drug cycles for individuals undergoing CPB was 8 (range, 1–26), and for those who underwent CPA, the median was 7 (range, 1–28). Of 246, 197 (80.1%) individuals discontinued CPB or CPA, primarily attributed to disease progression (55.8% for CPB vs. 44.2% for CPA, p = 0.001). Even though more patients developed disease progression in the CPB group but there was a significant delay in the time taken for disease to progress in this group which contributed to significantly longer PFS.
Fig. 1.
Flow diagram exhibiting the methods applied to identify objects to evaluate survival of cisplatin-paclitaxel chemotherapy plus BEV (CPB) versus cisplatin-paclitaxel chemotherapy alone (CPA) in postmenopausal women with previously untreated advanced cervical cancer (CC)
Table 1.
Patient demographics between groups
| Variable | CPB (n = 124) | CPA (n = 122) | p-value |
|---|---|---|---|
| Age (years) | 62.1 ± 7.59 | 62.2 ± 6.84 | 0.183a |
| BMI | 24.5 ± 5.31 | 25.6 ± 6.19 | 0.102a |
| Haemoglobin < 11 g/dLc, n (%) | 36 (29.0) | 32 (26.2) | 0.624b |
| Histology, n (%) | 0.439b | ||
| Squamous | 79 (63.7) | 72 (59.0) | |
| Adenocarcinoma | 34 (27.4) | 37 (30.3) | |
| Adenosquamous | 11 (8.9) | 13 (10.7) | |
| IVB cervical cancer, n (%) | 0.533b | ||
| Asymptomatic CNS metastasis | 45 (36.3) | 49 (40.2) | |
| Without CNS metastasis | 79 (63.7) | 73 (59.8) | |
| Duration of drug (months) | 17.8 ± 7.46 | 17.4 ± 7.63 | 0.219a |
| ECOG status, n (%) | 0.663b | ||
| 0d | 46 (37.1) | 42 (34.4) | |
| 1e | 78 (62.9) | 80 (65.6) | |
| GOG statusf, n (%) | 0.672b | ||
| 0f | 49 (39.5) | 45 (36.9) | |
| 1g | 75 (60.5) | 77 (63.1) | |
| No. of metastatic sites, n (%) | 0.445b | ||
| 3 | 38 (30.6) | 30 (27.8) | |
| > 3 | 71 (57.3) | 78 (62.0) | |
| Unknown | 15 (12.1) | 14 (10.2) |
aAnalysed using an independent samples t-test; bAnalysed using the Mann-Whitney U test;
cHaemoglobin level was calculated one week before the start of CPB or CPA; 0d, Fully active, able to carry on all pre-disease performance without restriction; 1e, Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light housework, office work; 0f: fully active; 1g: restricted in physically strenuous activities, but ambulatory. CPB cisplatin-paclitaxel chemotherapy plus bevacizumab, CPA cisplatin-paclitaxel chemotherapy alone, BMI body mass index, CNS central nervous system, ECOG Eastern Collaborative Oncology Group, GOG Gynecologic Oncology Group
Survival analysis
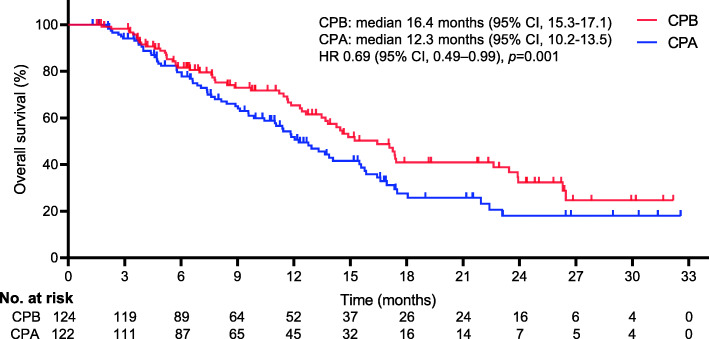
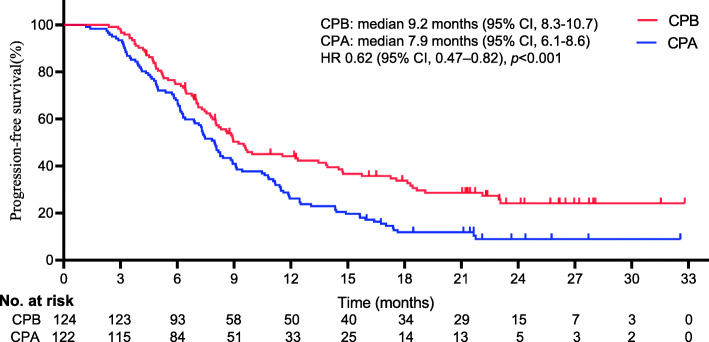
Figures 2 and 3 show the KM survival plot for the differences in OS and PFS between the 2 different regimens at the final follow-up, respectively. Throughout the follow-up period, 129 deaths were reported (52% [129/246]; 58 patients for CPB vs. 71 patients for CPA). A borderline noteworthy distinction was detected in the median OS between the two regimens (16.4 months [95% CI, 15.3–17.1] for CPB vs. 12.3 months [95% CI, 10.2–13.5] for CPA). The incorporation of BEV significantly improved the median OS compared with chemotherapy alone (HR 0.69, 95% CI, 0.49–0.99; p = 0.001). There was also a distinct difference in the median PFS between the two regimens (9.2 months [95% CI, 8.3–10.7] for CPB vs. 7.9 months for CPA (95% CI, 6.1–8.6) (HR 0.62, 95% CI, 0.47–0.82; p < 0.001).
Fig. 2.
Kaplan-Meier curves for overall survival. The median overall survival was 16.4 months (95% confidence interval [CI], 15.3–17.1) for CPB and 12.3 months (95% CI, 10.2–13.5) for CPA (HR 0.69, 95%CI, 0.49–0.99; p = 0.001). *The hazard ratio was calculated using a Cox proportional hazards model, with the age, BMI, haemoglobin < 11 g/dL, histology, IVB CC, duration of drug, performance scores, and number of metastatic sites used as covariates and therapy as the time-dependent factor
Fig. 3.
Kaplan-Meier curves for progression-free survival. The median progression-free survival was 9.2 months (95% confidence interval [CI], 8.3–10.7) for CPB and 7.9 months (95% CI 6.1–8.6) for CPA (HR 0.62, 95%CI, 0.47–0.82; p < 0.001). *The hazard ratio was calculated using a Cox proportional hazards model, with the age, BMI, haemoglobin < 11 g/dL, histology, IVB CC, duration of drug, performance scores, and number of metastatic sites used as covariates and therapy as the time-dependent factor
Adverse events
Of the 246 patients, 43 (25 [20.2%] receiving CPB vs. 18 [14.8%] receiving CPA; p = 0.265) had not documented persistent disease at the final follow-up. Table 2 demonstrates the frequency of AEs probably associated with CPB or CPA. Compared with CPB, CPA was associated with fewer AEs (9.9% vs. 12.0%, p < 0.001). There was a significant difference between the groups for hypertension grade ≥ 2 (25.0% receiving CPB vs. 4.1% receiving CPA, p < 0.001), but it was generally controllable. Additionally, significant differences were noted between the groups for neutropenia grade ≥ 4 (33.9% receiving CPB vs. 14.8% receiving CPA, p < 0.001) and thrombosis/embolism grade ≥ 3 (8.9% receiving CB vs. 2.5% receiving CA, p = 0.030). There were no significant differences in terms of genitourinary fistula grade ≥ 2, gastrointestinal fistula grade ≥ 2, gastrointestinal bleeding grade ≥ 3, or pain grade ≥ 2.
Table 2.
Adverse events
| Grade, n% | CPB (n = 124) | CPA (n = 122) | HR (95%) | p-value |
|---|---|---|---|---|
| ≥ 2 genitourinary fistula | 0 (0) | 0 (0) | NA | 1.000 |
| ≥ 2 gastrointestinal fistula | 0 (0) | 0 (0) | NA | 1.000 |
| ≥ 3 gastrointestinal bleeding | 0 (0) | 0 (0) | NA | 1.000 |
| ≥ 2 hypertension | 31 (25.0) | 5 (4.1) | 7.00 (4.52–6.02) | < 0.001* |
| ≥ 4 neutropenia | 42 (33.9) | 18 (14.8) | 4.00 (3.83–5.36) | < 0.001* |
| ≥ 3 thrombosis/embolism | 11 (8.9) | 3 (2.5) | 2.00 (1.48–3.71) | 0.030* |
| ≥ 2 pain | 38 (30.6) | 34 (27.9) | 3.00 (0.14–4.23) | 0.633 |
*Statistically significant. CPB cisplatin-paclitaxel chemotherapy plus bevacizumab, CPA cisplatin-paclitaxel chemotherapy alone, HR Hazard ratio, NA not applicable
Discussion
Findings from this retrospective study showed that the incorporation of BEV into cisplatin-paclitaxel chemotherapy led to significantly longer PFS times for postmenopausal women with previously untreated advanced CC, leading to significantly longer OS than those who received cisplatin-paclitaxel chemotherapy alone, with a controllable safety profile. The Kaplan–Meier curve for survival among these cases indicated an early advantage for patients receiving CPB that remained until final follow-up, with a difference in median OS of 4.1 months, which reached statistical significance.
The conclusion of the present study is consistent with the findings from a previous phase III trial [8], which assessed the effectiveness of BEV and nonplatinum combination chemotherapy in patients with recurrent, persistent, or metastatic CC. In this trial, the bevacizumab-containing regimen markedly improved the median OS compared to chemotherapy alone (17.0 months vs. 13.3 months; HR for death, 0.71; 98% CI, 0.54–0.95). Other clinical trials [5, 17] investigating the combined use of cisplatin-paclitaxel chemotherapy and the antiangiogenic agent BEV have shown a reduced hazard of disease progression, with median OS times ranging from approximately 16 to 18 months. The combination was effective and well tolerated in patients with advanced CC, which is currently recommended by the National Comprehensive Cancer Network as the standard of care for such patients [10]. The lack of effective therapies in advanced CC following the development of acquired resistance to conventional chemotherapy is a major clinical problem [5, 8]. The prognosis of these patients is still not favourable. Previous chemotherapy regimens have demonstrated a positive effect on metastatic CC; nevertheless, cisplatin-paclitaxel chemotherapy is still regarded as the preferred treatment option, which was established by the GOG 204 protocol [18].
VEGF contributes to the development of new tumour vasculature and is important for the survival and proliferation of cancer cells [10]. The expression of VEGF tends to be correlated with the biological aggressiveness of CC and is associated with poor survival [17, 19, 20]. Sequestration of VEGF using BEV when combined with chemotherapy was associated with improved survival among women with advanced CC [5, 20]. A phase II study (GOG-227C) [21] showed that BEV prevents tumour angiogenesis by blocking VEGF and was demonstrated to be active in recurrent CC. ESMO Clinical Practice Guidelines [22] indicated that the incorporation of BEV into cisplatin-paclitaxel chemotherapy is the preferred first-line regimen in advanced CC based on the balance between efficacy and toxicity profile.
Consistent with the GOG 240 [5], the current study showed that marked separations in median OS were observed (16.4 months [95% CI, 15.3–17.1] for CPB vs. 12.3 months [95% CI, 10.2–13.5] for CPA; HR 0.69, 95% CI, 0.49–0.99; p = 0.001). A retrospective study [23] of 52 patients with advanced CC who received the cisplatin-paclitaxel-BEV triplet reported a median OS of 15.3 months and a median PFS of 9.8 months. Recently, a retrospective observational study [24] involving 264 Chinese women with advanced CC who underwent cisplatin-paclitaxel-BEV triplet or cisplatin-paclitaxel chemotherapy alone showed that the cisplatin-paclitaxel-BEV triplet is associated with improved survival compared to cisplatin-paclitaxel chemotherapy alone (median OS: 540 days [95% CI, 483–597] for cisplatin-paclitaxel-BEV triplet vs. 357 days [95% CI, 264–450)] for cisplatin-paclitaxel chemotherapy alone; HR 1.21, 95% CI, 1.14–1.73; p = 0.002). A phase 3 trial (GOG 240) [1] showed that the cisplatin-paclitaxel-BEV triplet yields more significant improvement in survival than cisplatin-paclitaxel chemotherapy alone. An NRG Oncology/GOG study [5] of 390 female patients with advanced CC showed that improvements in survival were associated with BEV.
The efficacy of BEV has been revealed to be involved in the reconstruction of normal vasculature at the tumour site, initiating enhanced nutrient and oxygen supply while also escalating the delivery of cisplatin-paclitaxel drugs to the area occupied by the tumour [3, 25, 26]. VEGF signalling participates in several important physiologic processes (i.e., angiogenesis and blood pressure regulation) [3]. While blocking VEGF signalling may inhibit the progression of advanced CC, it can also initiate unintended effects because several other functional adjustments are achieved through VEGF signalling [27, 28]. BEV is directly related to the development of hypertension, which is a recognized effect of anti-VEGF therapy [3]. Since VEGF is indispensable and used to sustain vascular homeostasis, blocking the VEGF pathway can initiate endothelial dysfunction and hypertension [28]. The pathogenesis of this type of BEV-induced hypertension is not fully understood. One possible explanation [13, 29, 30] is that blocking VEGF results in a decrease in nitric oxide (NO) that dilates blood vessels.
Several important limitations should be recognized. First, this retrospective study had inherent shortcomings. Although this study included only postmenopausal women with previously untreated advanced CC, the relevant regression analysis can only control for measurable confounding factors. Residual confusion remains an important issue and may lead to underestimation of the harm associated with drug intervention. Second, when paclitaxel was administered at the maximum dose of 175 mg/m2/day, toxicity was escalated to a degree, which may result in a shorter medication cycle. Nonetheless, since the specifications of the drugs used by patients are uniform, this can avoid drug differences in the baseline data. Third, the current subjects were limited to postmenopausal women with previously untreated advanced CC, and thus, the results may not be generalizable to patients receiving routine treatment.
Conclusion
The results reported here may support the growing body of evidence demonstrating that the cisplatin-paclitaxel-BEV triplet combination is associated with increased survival benefit in postmenopausal women with previously untreated advanced CC. Furthermore, the safety and efficacy of long-term therapy with cisplatin-paclitaxel with and without BEV maintenance needs to be explored in postmenopausal women with previously untreated advanced CC.
Acknowledgements
Not applicable.
Abbreviations
- CC
Cervical cancer
- BEV
Bevacizumab
- VEGF
Vascular endothelial growth factor
- OS
Overall survival
- HR
Hazard ratio
- CPB
Cisplatin-paclitaxel chemotherapy plus BEV
- CPA
Cisplatin-paclitaxel chemotherapy alone
- ECOG
Eastern Collaborative Oncology Group
- GOG
Gynecologic Oncology Group
- CNS
Central nervous system
- PFS
Progression-free survival
- BMI
Body mass index
- CT
Computed tomography
- RECIST
Response Evaluation Criteria in Solid Tumours
- CI
Confidence interval
Authors’ contributions
GC, XL, WY, and MC performed the data collection and manuscript writing. WY, XL, and GC performed statistical analysis. GC and LD participated in the study design. All authors have read and approved the final manuscript.
Funding
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee (The First Affiliated Hospital, Sun Yat-sen University), and an exemption from informed consent was obtained from our responsible Investigational Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guanghua Chu and Xiangzhen Liu contributed equally to this work.
References
- 1.Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, DiSaia PJ, Copeland LJ, Creasman WT, Stehman FB, Brady MF, Burger RA, Thigpen JT, Birrer MJ, Waggoner SE, Moore DH, Look KY, Koh WJ, Monk BJ. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240) Lancet. 2017;390(10103):1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi CH, Chung JY, Kang JH, Paik ES, Lee YY, Park W, Byeon SJ, Chung EJ, Kim BG, Hewitt SM, Bae DS. Chemoradiotherapy response prediction model by proteomic expressional profiling in patients with locally advanced cervical cancer. Gynecol Oncol. 2020;157(2):437–443. doi: 10.1016/j.ygyno.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaman S, Leppänen VM, Alitalo K. Vascular endothelial growth factor signaling in development and disease. Development. 2018;145(14):dev151019. doi: 10.1242/dev.151019. [DOI] [PubMed] [Google Scholar]
- 4.Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L, Zeigenfuss S, Pruitt SK, Leary A. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2019;37(17):1470. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 5.Penson RT, Huang HQ, Wenzel LB, Monk BJ, Stockman S, Long HJ, Ramondetta LM, Landrum LM, Oaknin A, Reid TJA, Leitao MM, Method M, Michael H, Tewari KS. Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG oncology-gynecologic oncology group protocol 240) Lancet Oncol. 2015;16(3):301–311. doi: 10.1016/S1470-2045(15)70004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maluf FC, Leiser AL, Aghajanian C, Sabbatini P, Pezzulli S, Chi DS, Wolf JK, Levenback C, Loh E, Spriggs DR. Phase II study of tirapazamine plus cisplatin in patients with advanced or recurrent cervical cancer. Int J Gynecol Cancer. 2006;16(3):1165–1171. doi: 10.1136/ijgc-00009577-200605000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Al-Husein B, Abdalla M, Trepte M, DeRemer DL, Somanath PR. Antiangiogenic therapy for Cancer: an update. Pharmacotherapy. 2012;32(12):1095–1111. doi: 10.1002/phar.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ. Improved survival with Bevacizumab in advanced cervical Cancer. N Engl J Med. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazazi-Hyseni F, Beijnen JH, Schellens JHM. Bevacizumab. Oncologist. 2010;15(8):819–825. doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bizzarri N, Ghirardi V, Alessandri F, Venturini PL, Menada MV, Rundle S, Maggiore ULR, Ferrero S. Bevacizumab for the treatment of cervical cancer. Expert Opin Biol Ther. 2016;16(3):407–419. doi: 10.1517/14712598.2016.1145208. [DOI] [PubMed] [Google Scholar]
- 11.Skelton WP, Castagno J, Cardenas-Goicoechea J, Daily K, Yeung A, Markham MJ. Bevacizumab eligibility in patients with metastatic and recurrent cervical Cancer: a retrospective review. Clin Med Insights-Oncol. 2018;12:1179554918779587. doi: 10.1177/1179554918779587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigsby PW, Massad LS, Mutch DG, Powell MA, Thaker PH, McCourt C, Hagemann A, Fuh K, Kuroki L, Schwarz JK, Markovina S, Lin AJ, Dehdashti F, Siegel BA. FIGO 2018 staging criteria for cervical cancer: impact on stage migration and survival. Gynecol Oncol. 2020;157(3):639–643. doi: 10.1016/j.ygyno.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Plummer C, Michael A, Shaikh G, Stewart M, Buckley L, Miles T, Ograbek A, McCormack T. Expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving bevacizumab in the UK. Br J Cancer. 2019;121(2):109–116. doi: 10.1038/s41416-019-0481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edeline J, Boucher E, Rolland Y, Vauleon E, Pracht M, Perrin C, Le Roux C, Raoul JL. Comparison of tumor response by response evaluation criteria in solid tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118(1):147–156. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 15.Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D. Patient versus clinician symptom reporting using the National Cancer Institute common terminology criteria for adverse events: results of a questionnaire-based study. Lancet Oncol. 2006;7(11):903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 16.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Eskander RN, Tewari KS. Development of bevacizumab in advanced cervical cancer: pharmacodynamic modeling, survival impact and toxicology. Future Oncol. 2015;11(6):909–922. doi: 10.2217/fon.14.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, Benda J, Cella D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a gynecologic oncology group study. J Clin Oncol. 2009;27(28):4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tewari KS, Sill MW, Monk BJ, Penson RT, Long HJ, Poveda A, Landrum LM, Leitao MM, Brown J, Reid TJA, Michael HE, Moore DH. Prospective validation of pooled prognostic factors in women with advanced cervical Cancer treated with chemotherapy with/without Bevacizumab: NRG oncology/GOG study. Clin Cancer Res. 2015;21(24):5480–5487. doi: 10.1158/1078-0432.CCR-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee S. Bevacizumab in cervical cancer: a step forward for survival. Lancet. 2017;390(10103):1626–1628. doi: 10.1016/S0140-6736(17)31965-7. [DOI] [PubMed] [Google Scholar]
- 21.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of Bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2009;27(7):1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, Committee EG Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 23.Lee N, Kim SI, Lee M, Kim HS, Kim JW, Park NH, Song YS. Bevacizumab efficacy and recurrence pattern of persistent and metastatic cervical Cancer. In Vivo. 2019;33(3):863–868. doi: 10.21873/invivo.11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X, Liu J, Xiao L, Zhao M, Su T, Liu T, Han G, Wang Y. Cisplatin-based chemotherapy with or without bevacizumab for Chinese postmenopausal women with advanced cervical cancer: a retrospective observational study. BMC Cancer. 2020;20(1):381. doi: 10.1186/s12885-020-06854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol. 2010;117(3):497–504. doi: 10.1016/j.ygyno.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. 2015;20(2):166–175. doi: 10.1634/theoncologist.2014-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minion LE, Tewari KS. The safety and efficacy of bevacizumab in the treatment of patients with recurrent or metastatic cervical cancer. Expert Rev Anticancer Ther. 2017;17(3):191–198. doi: 10.1080/14737140.2016.1246187. [DOI] [PubMed] [Google Scholar]
- 29.Neagoe PE, Lemieux C, Sirois MG. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J Biol Chem. 2005;280(11):9904–9912. doi: 10.1074/jbc.M412017200. [DOI] [PubMed] [Google Scholar]
- 30.Carpini JD, Karam AK, Montgomery L. Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis. 2010;13(1):43–58. doi: 10.1007/s10456-010-9163-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.