Abstract
In this study, we investigated the bioactive potential (antibacterial and antioxidant), anticancer activity and detailed phytochemical analysis of Selaginella repanda (S. repanda) ethanolic crude extract for the very first time using different in vitro approaches. Furthermore, computer-aided prediction of pharmacokinetic properties and safety profile of the identified phytoconstituents were also employed in order to provide some useful insights for drug discovery. S. repanda, which is a rich source of potent natural bioactive compounds, showed promising antibacterial activity against the tested pathogenic bacteria (S. aureus, P. aeruginosa, E. coli and S. flexneri). The crude extract displayed favorable antioxidant activity against both 2,2-diphenyl-1-picrylhydrazyl (DPPH) (IC50 = 231.6 μg/mL) and H2O2 (IC50 = 288.3 μg/mL) molecules. S. repanda also showed favorable and effective anticancer activity against all three malignant cancer cells in a dose/time dependent manner. Higher activity was found against lung (A549) (IC50 = 341.1 μg/mL), followed by colon (HCT-116) (IC50 = 378.8 μg/mL) and breast (MCF-7) (IC50 = 428.3 μg/mL) cancer cells. High resolution-liquid chromatography–mass spectrometry (HR-LC–MS) data of S. repanda crude extract revealed the presence of diverse bioactive/chemical components, including fatty acids, alcohol, sugar, flavonoids, alkaloids, terpenoids, coumarins and phenolics, which can be the basis and major cause for its bioactive potential. Therefore, achieved results from this study confirmed the efficacy of S. repanda and a prospective source of naturally active biomolecules with antibacterial, antioxidant and anticancer potential. These phytocompounds alone with their favorable pharmacokinetics profile suggests good lead and efficiency of S. repanda with no toxicity risks. Finally, further in vivo experimental investigations can be promoted as probable candidates for various therapeutic functions, drug discovery and development.
Keywords: Selaginella repanda, breast cancer, lung cancer, colon cancer, phytochemistry, natural product, phytomedicine, pharmacokinetics, ADMET
1. Introduction
From decades, plants are well conceded as a source of medicine against different kinds of human ailments [1,2,3]. Several examples of plant-based drugs include nicotine (antimicrobial), nordihydroguaiaretic acid (antioxidant), vinblastine (anticancer), aescin (anti-inflammatory), acetyldigoxin (cardiotonic), l-dopa (antiparkinsonism), etc. In spite of the discovery of innumerable drugs of plant origin, the exploration of novel bioactive compounds is yet requisite to enhance the range access and to search less toxic and more efficacious drugs [4,5,6]. Medicinal and economic importance of higher plants, specifically angiosperms, has been explored thoroughly. However, lycophytes and ferns have been woefully overlooked. Most humanity presumes that there is a little usage of lycophytes and ferns. Though, these plants have delineated plenty of health-related benefits to humanity since ancient times. Their uses are urged in the Ayurvedic (Sushruta, Charka, Samhita), Unani, homeopathic and other systems of medicines. They exert influence on millions of human lives as conventional treatments for various diseases like, burn, cold, ascarid diseases, trauma bleeding, diarrhea and others [7].
The cosmopolitan genus Selaginella also acknowledged as a “spike moss” possessing around 700–750 species distributed around the globe. The members of the Selaginella are well known for their uses in conventional folk medicine, food, handicrafts and as ornaments. The Selaginella plants are usually used by the tribal community to cure fever, jaundice, hepatic disorders, cirrhosis, diarrhea, cholecystitis, sore throat, cough of lungs, promote blood circulation, remove blood stasis and stops external bleeding after trauma and after separation of the umbilical cord [8]. Few species of Selaginella such as, S. tamariscina, S. lepidophylla, S. chrysocaulos, S. bryopteris, S. labordei and S. moellendorffii have been reported for their in vitro antimicrobial, antiviral, antidiabetic, antimutagenic, anti-inflammatory, antinociceptive, antispasmodic and anticancer potentials due to the high content of different phytochemicals, such as flavonoids, phenylpropanoids, steroids, pigments, oxygen heterocycle, lignans, coumarins, quinoids, chromones, benzenoids, carbohydrates and alkaloids [9,10,11,12,13,14,15,16,17,18]. However, very few information related to S. repanda phytochemistry is available, though it has been reported as an important ethnomedicinal fern.
Therefore, this study aims to evaluate the bioactivities (antibacterial, antioxidant and anticancer activity against human lung, breast and colon cancer cell lines) of S. repanda for the first time. In order to evaluate the potency and bioactive potential, S. repanda crude extract was also analyzed by high resolution-liquid chromatography–mass spectrometry (HR-LC–MS) in detail to identify the phytochemical constituent’s being the reason for its bioactivity. Obtained results will support the further in vivo studies for the possible therapeutic usage of S. repanda against various diseases/ailments and in the treatment of metastatic cancer.
2. Results and Discussion
Medicinal plants have been utilized chiefly as ethnic remedies against various diseases since ancient time. They are extensively contemplated as a source of novel phytochemicals with bioactive potential. In order to treat a variety of diseases, these phytomedicines can also lead in drug discoveries or itself become a potential drug. Hence, it is very essential to determine and estimate the bioactive potential of ethnomedicinal plants by particularizing the phytochemistry and developing them as a potent source for therapeutic agents [19,20,21]. Pteridophytes ethnomedicinal nature makes them as a traditional medicine to cure several complicated health conditions [22,23,24]. Therefore, in the presented work, we evaluated the antibacterial, antioxidant and anticancer properties of S. repanda.
2.1. Antibacterial Potential of S. repanda
In recent times, almost all developing and developed countries are going through with a global problem of antibiotic resistance, which is continuously challenging the global healthcare sector. Multidrug-resistant pathogens are emerging and spreading in such a way that it continues to diminish the current antimicrobial therapies. This demands a search for novel antimicrobial substances, preferably from natural sources. Plants are one such example consisting of a variety of bioactive compounds with known therapeutic properties [3]. The microdilution method was used to study the antagonistic potential of S. repanda crude extract against pathogenic bacterial strains S. flexneri, S. aureus, E. coli and P. aeruginosa. Results revealed that S. repanda showed significant antagonistic activity against all the four tested bacterial strains, and are presented in Table 1.
Table 1.
Antibacterial activity of S. repanda crude extract.
| Bacterial Strain |
S. repanda Crude Extract (µg/mL) (MIC) |
Chloramphenicol (µg/mL) (MIC) |
Negative Control (85% Ethanol) |
|---|---|---|---|
| E. coli | 31.25 | 7.812 | - |
| S. aureus | 62.5 | 15.625 | - |
| P. aeruginosa | 125 | 31.25 | - |
| S. flexneri | 250 | 62.5 | - |
The antimicrobial activity of different species of Selaginella was evaluated against various human pathogenic bacteria. The crude extract of S. bryopteris was reported for its antibacterial activity against different Gram-negative and Gram-positive bacteria such as E. coli, E. faecalis, C. tropicalis, S. aureus, C. albicans, C. krusei and K. pneumonia [25]. S. equalifolia and S. involvens has been reported to show antimicrobial activity against different poultry pathogens namely, Klebsiella, Salmonella, Staphylococcus, Proteus and Bacillus [26]. S. inaequalifolia showed good antagonistic activity against S. aureus, E. coli and C. albicans [27]. S. convoluta showed significant antibacterial potential against B. cereus, E. coli, S. enterica, S. marcescens, K. pneumoniae, S. flexneri, E. faecalis and S. aureus [28]. S. tamariscina is known to possess potent activity against oral bacterial pathogens such as P. gingivalis, P. intermedia, S. mutans, S. sobrinus, S. gordonii, F. nucleatum, S. sanguinis, S. anginosus, S. ratti, A. actinomycetemcomitans, S. parasanguinis, S. criceti and S. downei [29]. Our study is also in line with previous antibacterial studies and showed that S. repanda possess antibacterial potential against various human pathogenic bacteria.
2.2. Antioxidant Potential of S. repanda
Free radicals are naturally formed in our body and perform many cellular processes [30]. At a higher concentration, free radicals can be dangerous and can damage the important constituents of the cell, which may include cell membrane, proteins, DNA, etc. Damage to DNA, in particular, may lead to cancer or other severe health conditions. However, in order to prevent this type of cellular damage, antioxidants play an essential role. They are generally described by “mopping up” free radicals, which means they are able to neutralize the electrical charge and can prevent the free radical from receiving electrons from other molecules [30]. It has been reported that plant extract or naturally extracted compounds having antioxidant activity, exhibits anticancer activity in a defined mechanism.
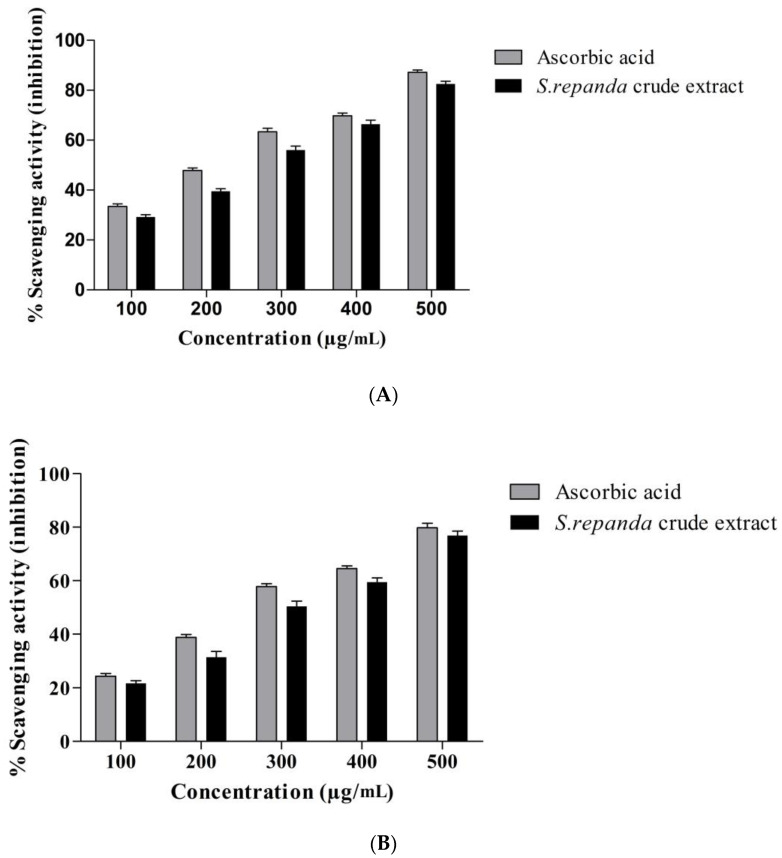
S. repanda antioxidant potential was analyzed against DPPH and H2O2 molecules in comparison to ascorbic acid. The results of antioxidant activity displayed that S. repanda crude extract exhibited remarkable free radical scavenging activity, both against DPPH and H2O2 molecules in a dose dependent manner. The scavenging capacity against DPPH free radicals was higher than H2O2 molecules. The IC50 values of crude extract of S. repanda for DPPH and H2O2 were 231.6 μg/mL and 288.3 μg/mL, respectively (Figure 1A,B). The antioxidant potential of different species of Selaginella have been evaluated by different methods; from which, most are based on the determination of free radical scavenging activity. Commonly used methods are, ABTS, DPPH, superoxide anion radical scavenging assays and total phenolic content. Total phenolic content of S. repanda dried powder was found to be 6.51 ± 0.71 mg gallic acid equivalents/g dry matter. Indian Sanjeevani (S. bryopteris) is well known for its protective effect against various stress-induced conditions [31]. The crude extract of S. tamariscina have strong antioxidant property, as its extract can reduce blood sugar levels and also able to act as a lipid peroxide and increases insulin serum [32]. In one study, the aqueous extract of S. involvens, S. delicatula and S. wightii also displayed in vitro lipid peroxidation and varying levels of hydroxyl radical scavenging activity. The 50% inhibition (EC50) for in vitro lipid peroxidation of S. wightii, S. delicatula and S. involvens was 76.6 ± 4, 38.2 ± 1.2 and 2.1 ± 0.1, respectively. Moreover, flavonoids obtained from the S. doederleinii also possess very strong free radical scavenging activity [33]. Similar results were found in our study. S. repanda displayed potent antioxidant activity, indicating its protective role against free radicals.
Figure 1.
Antioxidant activity of ascorbic acid (standard) and S. repanda crude extract against (A). DPPH molecules. (B). H2O2 molecules. Error bars indicate SDs (±standard deviation) of three independent experiments (p < 0.05).
2.3. Anticancer Potential of S. repanda
Cancer is the main health burden in developing and developed countries, as it is the second leading cause of deaths globally. Different types of treatments are available for the therapy, depending on the stage of cancer and type. Radiotherapy and surgery are efficacious in the treatment of cancer at the earlier stage, while chemotherapy was used when the tumor reaches the stage of metastasis. However, the high cost of treatments is associated with the side effects, which accounted for the use of herbal products for the treatment of different types of cancers. Over the past decade, natural products perceived high attention because of their capability as novel therapeutic and preventive agents. About 60% of all orthodox anticancer drugs are directly or indirectly derived from plants [34], which imply that medicinal plants have the ability in leading to the discovery of novel drugs.
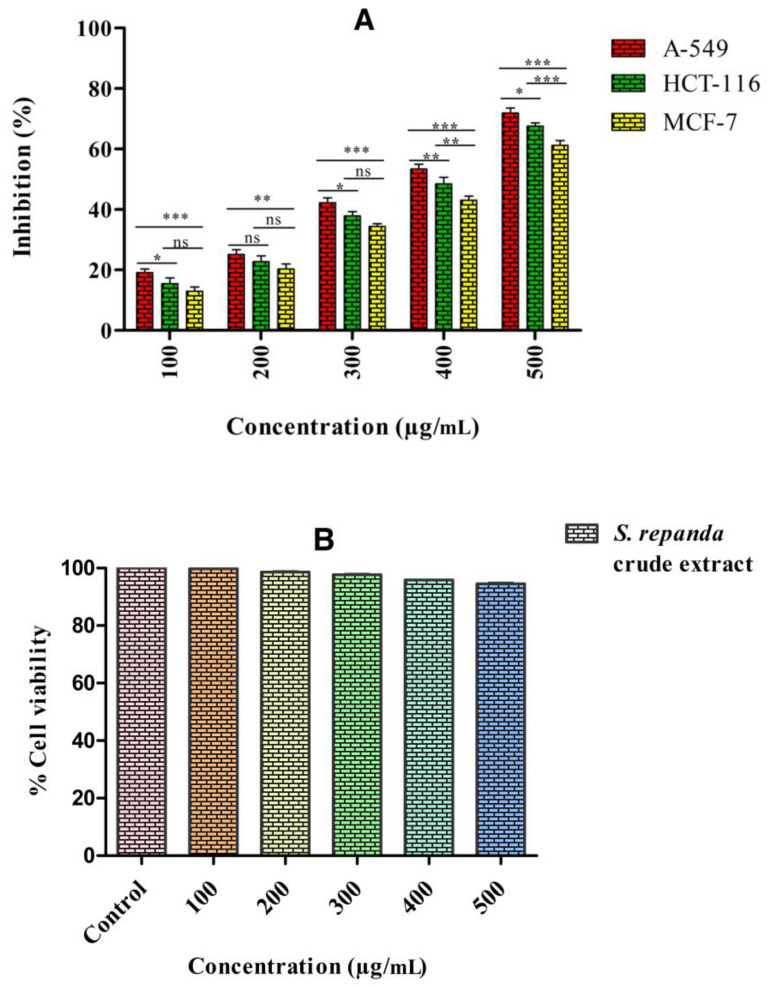
Selaginella species have different groups of chemical compounds, which are well known to have a wide-range of biological actions and are a potential source for finding the novel anticancer drugs. As many of the phytochemical compounds obtained from few of these species possess strong cytotoxic activity against various cancer cell lines. S. repanda crude extract was evaluated for its anticancer potential by MTT assay against three different human cancer cell lines, i.e., breast (MCF-7), colon (HCT-116) and lung (A549). Our results revealed that, proliferation of all the three cell lines were inhibited by S. repanda in a dose dependent manner, compared to fluorouracil, which was used as the positive control. The crude extract of S. repanda showed the highest growth inhibitory activity against A549 cells with IC50 value of 341.1 μg/mL, followed by HCT-116 cells (378.8 μg/mL) and MCF-7 cells (428.3 μg/mL) (Figure 2A). Cytotoxicity test of S. repanda crude extract against non-cancerous human normal colon cells (CRL-1831) was also performed and results revealed no cytotoxicity to normal cells (Figure 2B).
Figure 2.
(A) Anticancer activity against three different human cancer cell lines, i.e., breast (MCF-7), colon (HCT-116) and lung (A549). (B) Cytotoxicity of S. repanda crude extract against human normal colon cells (CRL-1831). Error bars indicate SDs (± standard deviation) of three independent experiments. NS > 0.05, * p < 0.05, ** p < 0.005, *** p < 0.0005
One study revealed the anticancer effect of ethanolic and aqueous extracts of S. doederleinii using the brine shrimp lethality test against two cancer cell lines MDAMB231 (breast) and HepG2 (liver). As a result, after 24 h of exposure to 50% lethal concentration (LC50) in the brine shrimp lethality test was found to be >1000 μg/mL. MDAMB231 and HepG2 were found to be the most susceptible with the treatments of ethanol (LC50 = 306 μg/mL) and aqueous (LC50 = 329 μg/mL) extracts of S. doederleinii, respectively [35]. The crude extract of S. tamariscina also showed potent anticancer activity against different cancer cell lines. It was found to decrease the metastasis, expression of MMP-2 and 9 (matrix metalloproteinase) and urokinase plasminogen activator in A549 cells and Lewis lung carcinoma [36]; inhibits nucleus antigen cell from stomach epithelium [37]; inhibits gastric cancer cells [37] and induce apoptosis via blockade of fatty acid synthesis in breast cancer [38]; inhibits leukemia cancer cells HL-60 and U937 [39];. Apart from this, amentoflavone was extracted from the crude extract of S. tamariscina and its anticancer efficacy was screened against five different cancer cells, including HL-60 (human leukemia cells), HeLa (human cervical carcinoma cells), PANC-1 (human pancreatic cancer cells), MCF-7 (human breast cancer cells) and BEL-7402 (human hepatoma carcinoma cells). The extract was found to be efficient in the inhibition of the proliferation of all cells with a remarkable inhibition of HL-60 [39].
The cytotoxic effect and apoptosis induction potential of hexane, methylene chloride, ethyl acetate and butanol extracts of S. plana was performed against MCF-7 cells. Different crude extracts of S. plana displayed inhibition of MCF-7 cells with an IC50 value of 30 μg/mL, 19 μg/mL, 24 μg/mL and 2 μg/mL respectively. Butanol crude extract was found as the highest cytotoxic and apoptotic induction against MCF-7 cancer cells [40]. The cytotoxic and apoptosis activity of three different crude extracts (ethyl acetate, ethanol and aqueous) of S. uncinata, S. tamariscina, S. remotifolia, S. delicatula, S. moellendorfii, S. pulvinata and S. labordei were evaluated using BEL-7402, HT-29 and HeLa cells. In results, S. labordei, S. tamariscina and S. uncinata had a higher inhibition of Bel-7402 and HeLa cells, whereas, S. moellendorfii had moderate inhibition, but S. remotifolia and S. pulvinata had almost no inhibitory activities. The major bioactive compounds responsible for the inhibition for cancer cells were bioflavonoids, detected in the ethyl acetate extracts. Moreover, the efficacy of all three extracts of all the plants on cell inhibition and apoptosis were not the same, they were highly efficient on HeLa cells than HT-29 cells [41]. Our results also revealed similar data, S. repanda possessing anticancer potential against three malignant lineages, HCT-116, MCF-7 and A549 cell lines. Though, all the three cell lines have high metastatic potential, S. repanda was found to inhibit the proliferation of all three malignant cells in a time and concentration dependent manner.
2.4. Identification of Phytochemical Compounds of S. repanda Using HR-LC–MS
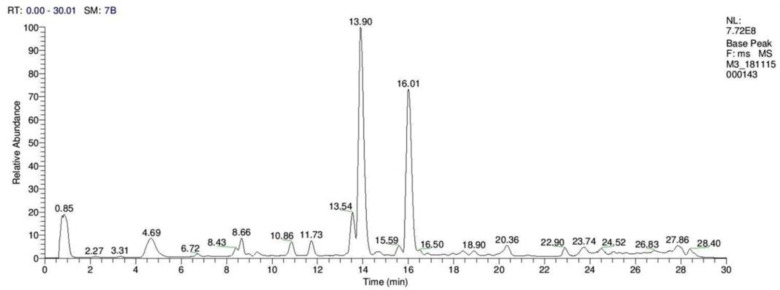
Different classes of phytochemicals are omnipresent in plants and have been linked with diverse biological activities. This study revealed the presence of diverse groups of chemical compounds, which are known to possess a broad range of bioactivities. After the evaluation of antibacterial, antioxidant and anticancer activities, a comprehensive phytochemical analysis from crude extract of S. repanda was carried out via UHPLC-PDA-ESI–MS/MS. Chromatogram was obtained with both positive and negative run and different types of phytochemicals were identified (Figure 3). Different classes of metabolites such as sugars, amino acids, vitamins, alkaloids, flavonoids, terpenoids, phenols, etc., were detected (Figure 4, Figure 5 and Figure 6).
Figure 3.
High resolution-liquid chromatography–mass spectrometry (HR-LC–MS) spectrum of S. repanda crude extract.
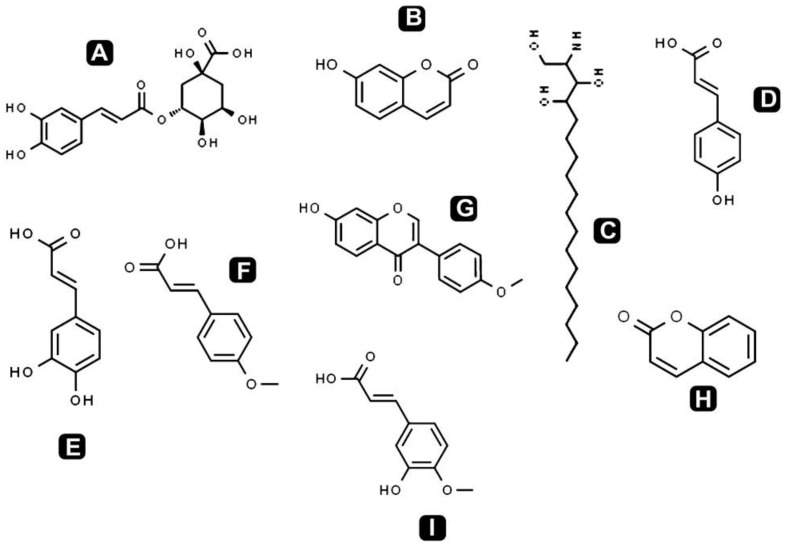
Figure 4.
Chemical structures of the identified phenol compounds in S. repanda crude extract. A. Chlorogenic acid B. 7-Hydroxycoumarine C. 2-amino-1,3,4-octadecanetriol D. 4-coumaric acid E. Caffeic acid F. 4-Methoxycinnamic acid G. Formononetin H. Coumarin I. Isoferulic acid.
Figure 5.
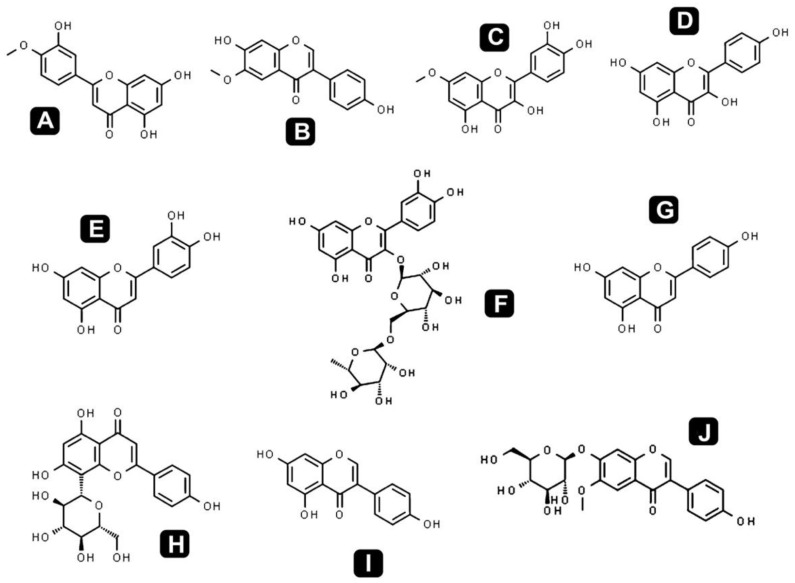
Chemical structures of the identified flavonoid compounds in S. repanda crude extract. A. Diosmetin B. Glycitein C. Rhamnetin D. Kaempferol E. Luteolin F. Rutin G. Apigenin H. Vitexin I. Genistein J. Glycitin.
Figure 6.
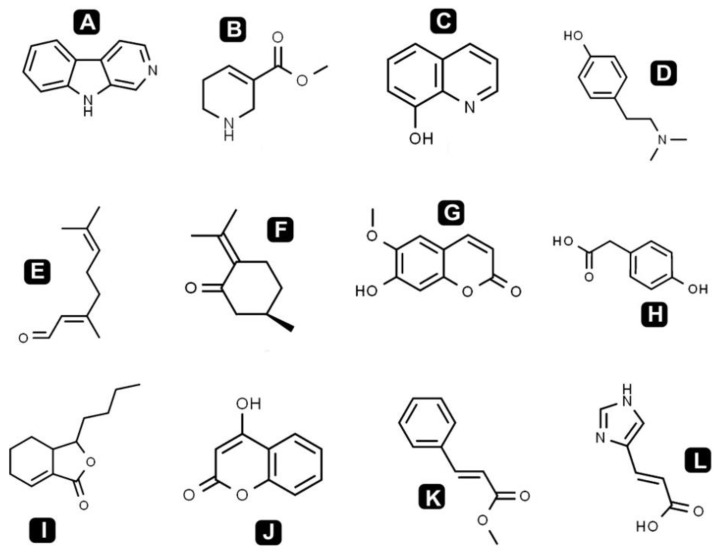
Chemical structures of the identified alkaloids, terpenoids, coumarins, benzenoids, esters and monocarboxylic acid compounds in S. repanda crude extract. A. Norharman B. Guvacoline C. 8-Hydroxyquinoline D. Hordenine E. Citral F. Pulegone G. Scopoletin H. 4-Hydroxyphenylacetic acid I. Sedanolide J. 4-Hydroxycoumarine K. Methylcinnamate L. Urocanic acid.
Flavonoids and natural phenolic acids are one of the most prevalent and pharmacologically active groups of plant secondary metabolites. In this study, S. repanda crude extract revealed the presence of phenols like coumarin, chlorogenic acid, 7-hydroxycoumarine, caffeic acid, 4-methoxycinnamic acid, isoferulic acid, formononetin, 2-amino-1,3,4-octadecanetriol and 4-coumaric acid. The other main flavonoids identified in the present study were rutin, vitexin, quercetin, quercetin-3β-d-glucoside, kaempferol, apigenin, luteolin, rhamnetin, glycitein, diosmetin and genistein. They are well known for their potential therapeutic applications including antioxidant, anti-inflammatory, anticancer, cardioprotective effects, etc. These compounds play a crucial role in prevention of cancer through various mechanisms at the molecular level. This may include impeding the signaling pathways, migration, differentiation and proliferation inhibition, gene regulation, carcinogen metabolism, induction of apoptosis via arresting cell cycle, etc. [42].
Alkaloids are nitrogenous compounds, which are widely distributed from prokaryotes to eukaryotes and are well-known for their different biological activities like anti-microbial, anti-HIV, anticancer and antiparasitic [43]. In the present study, 8-hydroxyquinoline, norharman, hordenine and guvacoline were identified. Two terpenoids, citral and pulegone were also identified from the crude extract of S. repanda. Terpenoids possess diverse biological activities including antimalarial, antioxidant, antibacterial, antiviral, anticancer and anti-inflammatory. They are also used for the treatment of various diseases [20]. Apart from this, coumarins, different amino acids, fatty acids, sugars and sugar alcohol were also detected. Detailed list of identified compounds with their mass (m/z), retention time and bioactivities are presented in Table 2.
Table 2.
Profiling of bioactive compounds present in S. repanda crude extract with their biological activity.
| Compound | Formula | Class of Phytochemical | m/z | RT (min) |
Mass | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Choline | C5H13N O | essential nutrient (vitamin) | 111.4 | 0.798 | 103.09988 | - | - |
| α-Lactose | C12H22O11 | sugar | 349.9 | 0.839 | 342.11521 | - | - |
| Acetylcholine | C7H15 NO2 | essential nutrient (vitamin) | 149.7 | 0.850 | 145.11 | - | - |
| Betaine | C5H11NO2 | amino acid | 122.2 | 0.935 | 117.07901 | Antimicrobial, antioxidant, antiangiogenesis | [44] |
| l(-)-Carnitine | C7H15NO3 | amino acid derivative | 157.6 | 0.93 | 161.10489 | - | - |
| d-Glucosamine | C6 H13 NO5 | amino sugar | 183.3 | 0.946 | 179.079 | Antitumor | [45] |
| l-Pyroglutamic acid | C5H7NO3 | amino acid | 133.1 | 1.04 | 129.0425 | - | - |
| l-Norleucine | C6H13NO2 | amino acid | 136.5 | 1.134 | 131.09453 | Antimicrobial | [46] |
| l-Phenylalanine | C9H11NO2 | amino acid | 164.2 | 1.375 | 165.07883 | Antioxidant, anticancer | [47] |
| Guvacoline | C7H11NO2 | pyridine alkaloid | 145.2 | 1.539 | 141.07878 | - | - |
| Maltol | C6H6O3 | sugar | 130.4 | 2.278 | 126.03161 | Antioxidant | [48] |
| 8-Hydroxyquinoline | C9H7NO | alkaloid | 153.2 | 2.965 | 145.05255 | Antimicrobial | [49] |
| 4-Hydroxyphenylacetic acid | C8H8O3 | benzenoid | 156.3 | 3.314 | 152.04714 | Antimicrobial, antioxidant | [50] |
| 3-Methylcrotonylglycine | C7H11NO3 | amino acid | 152.6 | 3.325 | 157.0737 | - | - |
| Coumarin | C9H6O2 | phenol | 148.6 | 3.784 | 146.0365 | Antibacterial, antibiofilm | [51] |
| Kynurenic acid | C10H7NO3 | quinoline carboxylic acid | 181.7 | 3.823 | 189.04239 | Anticancer | [52] |
| Chlorogenic acid | C16H18O9 | phenol | 360.5 | 4.646 | 354.09435 | Anticancer, antioxidant, antibacterial, antiviral, antiobesity and anti-inflammatory | [53] |
| 7-Hydroxycoumarine | C9H6O3 | phenol | 169.4 | 4.651 | 162.0314 | Antioxidant, antinociceptive, anti-inflammatory, antitumor, immunomodulator | [54,55] |
| Caffeic acid | C9H8O4 | phenol | 186.2 | 4.692 | 180.04181 | Antioxidant, anti-inflammatory, anticancer and antineoplastic | [56] |
| Pulegone | C10H16O | terpenoid | 158.9 | 4.94 | 152.11989 | Antibacterial | [57] |
| Citral | C10H16O | terpenoid | 145.8 | 5.575 | 152.11989 | - | - |
| Scopoletin | C10H8O4 | coumarin | 196.3 | 5.969 | 192.04198 | Anticancer, antimicrobial | [58] |
| Isoferulic acid | C10H10O4 | phenol | 198.6 | 6.367 | 194.05762 | Antioxidant, antibacterial | [59] |
| Methyl cinnamate | C10H10O2 | cinnamic acid ester | 164.5 | 6.458 | 162.06775 | Antimicrobial, anticancer | [60] |
| 4-Hydroxycoumarin | C9H6O3 | benzopyrone | 158.8 | 6.568 | 162.0314 | Antibacterial, antioxidant, antitumor, anti-inflammatory | [56] |
| Norharman | C11H8N2 | alkaloid | 164.7 | 6.725 | 168.06847 | Antimicrobial | [61] |
| 4-Methoxycinnamic acid | C10H10O3 | phenol | 186.2 | 7.754 | 178.06275 | Antibacterial | [62] |
| Rutin | C27H30O16 | flavonoid | 615.4 | 8.291 | 610.15239 | Antimicrobial, antioxidant, anticancer, anti-inflammatory, antidiabetic and antiallergic | [63] |
| Vitexin | C21H20O10 | flavonoid | 436.8 | 8.319 | 432.10497 | Antimicrobial, antioxidant, antitumor | [63] |
| Quercetin | C15H10O7 | flavonoid | 308.6 | 8.414 | 302.04192 | Antioxidant, anti-inflammatory, antimicrobial, anticancer, antidiabetic | [64] |
| Quercetin-3β-d-glucoside | C21H20O12 | flavonoid | 260.3 | 8.438 | 464.09465 | Antioxidant | [65] |
| α-Pinene-2-oxide | C10H16O | terpenoid | 148.9 | 8.494 | 152.11989 | - | - |
| Sedanolide | C12H18O2 | isobenzofuran | 199.5 | 8.578 | 194.13039 | Anticancer, antioxidant | [66] |
| Kaempferol | C15H10O6 | flavonoid | 294.6 | 8.662 | 286.04726 | Antimicrobial, antioxidant, anticancer, anti-inflammatory, antidiabetic, antiallergic, antiosteoporotic, anxiolytic, analgesic, and antiallergic | [67] |
| Kuromanin | C21H20O11 | chloridis | 458.1 | 8.976 | 448.09996 | Anticancer | [68] |
| Cycloheximide | C15H23NO4 | 274.5 | 10.281 | 281.16216 | Antibacterial | [69] | |
| (-)-Caryophyllene oxide | C15H24O | epoxide | 228.4 | 10.861 | 220.18227 | Antioxidant, anticancer | [70] |
| Glycitin | C22H22O10 | isoflavone | 442.2 | 11.088 | 446.12065 | Antioxidant, antibacterial | [71] |
| Apigenin | C15H10O5 | flavone | 272.4 | 11.177 | 270.05235 | Anticancer, antibacterial, antioxidant | [72] |
| Luteolin | C15H10O6 | flavonoid | 278.6 | 11.735 | 286.04723 | Antioxidant, anticancer and anti-inflammatory | [73] |
| Formononetin | C16H12O4 | phenol | 265.3 | 12.157 | 268.07329 | Antioxidant, anticancer | [74] |
| Rhamnetin | C16H12O7 | flavonoid | 311.2 | 12.452 | 316.05766 | Antioxidant, anti-inflammatory, cardioprotective, anticancer | [75] |
| Glycitein | C16H12O5 | flavonoid | 288.9 | 13.477 | 284.06807 | - | - |
| 2-Amino-1,3,4-octadecanetriol | C18H39NO3 | phenol | 311.5 | 13.499 | 317.29231 | - | - |
| Diosmetin | C16H12O6 | flavonoid | 294.3 | 13.534 | 300.06279 | Antioxidant | [76] |
| Genistein | C15H10O5 | isoflavone | 275.3 | 13.542 | 270.05214 | Antimicrobial, antioxidant, anticancer | [77] |
| Valine | C5H11NO2 | amino acid | 118.4 | 13.901 | 117.07901 | - | - |
| 2-Arachidonoyl glycerol | C23H38O4 | fatty acid derivative | 371.0 | 16.012 | 378.27635 | Antimicrobial | [78] |
| 4-Coumaric acid | C9H8O3 | phenol | 168.2 | 18.265 | 164.04718 | Antibacterial, antioxidant, antitumor, antimutagenic | [79,80] |
| Arachidonic acid | C20H32O2 | polyunsaturated fatty acid | 301.8 | 18.601 | 304.2397 | - | - |
| Hexadecanamide | C16H33NO | fatty acid amide | 250.4 | 19.849 | 255.25575 | - | - |
| Oleamide | C18H35NO | fatty acid | 286.1 | 20.177 | 281.27135 | Antimicrobial, anticancer | [81] |
| Hordenine | C10H15NO | alkaloid | 167.2 | 23.071 | 169.23958 | - | - |
| Urocanic acid | C6H6N2O2 | monocarboxylic acid | 131.2 | 24.845 | 138.04267 | Antioxidant, anticancer | [82] |
Therefore, our data suggested that the presence of these diverse biomolecules in S. repanda crude extract is the major reason for its antibacterial, antioxidant and anticancer activities. Our results provide an opportunity to explore further this medicinally treasured pteridophyte. Moreover, for the first time we studied that the S. repanda crude extract had a strong dose dependent cytotoxicity against three malignant cancer cell lines (HCT-116, MCF-7 and A549). Further studies are needed in order to demonstrate the efficacy and potency of specific compound in the crude extract, which might be responsible for the particular biological activity.
2.5. Pharmacokinetic and Toxicity (ADMET) Profiles of Identified Phytoconstituents from S. repanda Ethanolic Crude Extract
It is well known that good drug activity may be destroyed due to inadequate ADMET (absorption, distribution, metabolism, excretion and toxicity) properties. In addition, unwanted pharmacokinetics and toxicity are important reasons for the failure of drug discovery in the clinical phase, which is very costly. In order to decide, the possibility of S. repanda ethanolic crude extract to become or not become a good candidate for suitable drug, ADMET parameters have been assessed using in silico tools (Table 3). Interestingly, all identified phytoconstituents were found to meet the Lipinski’s rule of five, and some of them such as hordenine, 4-coumaric acid and Diosmetin follows also Ghose, Veber and Egan filters with most of them displaying a good bioavailability score (55–85%). The solubility as an important feature for the absorption of the molecule and its distribution in the body given by the values of aqueous solubility indicates most of the compounds were highly soluble in water and the others are soluble.
Table 3.
Absorption, distribution, metabolism, excretion and toxicity (ADMET) properties of some top identified phytocompounds.
| Entry | 22 | 33 | 37 | 53 |
|---|---|---|---|---|
| Druglikeness | ||||
| Lipinski | Yes | Yes | Yes | Yes |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 |
| Absorption | ||||
| Water solubility | −2.504 | −3.068 | −4.321 | −1.219 |
| Caco2 permeability | 1.184 | 1.616 | 1.414 | 1.587 |
| Intestinal absorption (human) | 95.277 | 96.423 | 95.669 | 93.396 |
| Skin Permeability | −2.944 | −2.237 | −3.061 | −2.506 |
| P-glycoprotein substrate | No | No | No | No |
| P-glycoprotein I inhibitor | No | No | No | No |
| P-glycoprotein II inhibitor | No | No | No | No |
| Distribution | ||||
| VDss (human) | 0.034 | 0.31 | 0.564 | 0.887 |
| BBB permeability | −0.299 | 0.591 | 0.647 | -0.083 |
| CNS permeability | -2.32 | −2.511 | −2.521 | −1.75 |
| Metabolism | ||||
| CYP2D6 substrate | No | No | No | Yes |
| CYP3A4 substrate | No | No | No | No |
| CYP1A2 inhibitior | Yes | No | Yes | No |
| CYP2C19 inhibitior | No | No | Yes | No |
| CYP2C9 inhibitior | No | No | Yes | No |
| CYP2D6 inhibitior | No | No | No | No |
| CYP3A4 inhibitior | No | No | No | No |
| Excretion | ||||
| Total Clearance | 0.73 | 1.356 | 0.905 | 0.907 |
| Renal OCT2 substrate | No | No | No | Yes |
| Toxicity (Compounds number) | ||||
| AMES toxicity | 3 (10, 26, 27) | |||
| Hepatotoxicity | 3 (9, 25, 50) | |||
| hERG I inhibitors | No | |||
| Skin Sensitisation | 14 (2, 4, 5, 10, 20, 24, 32, 33, 37, 44, 48, 50, 51, 52) | |||
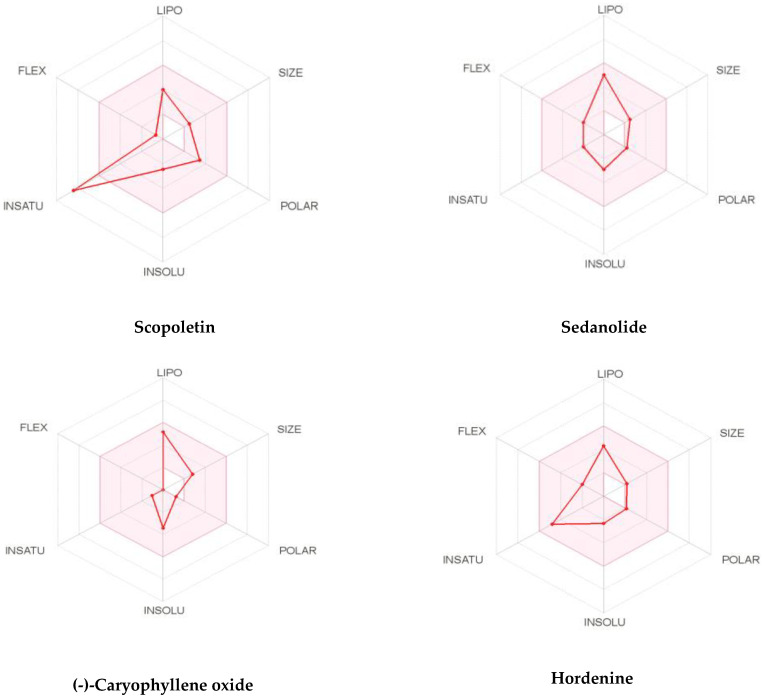
22: Scopoletin, 33: Sedanolide, 37: (-)-Caryophyllene oxide, 53: hordenine. VDss: volume of distribution at steady state; BBB: brain blood barrier; CNS: central nervous center; CYP: cytochrome P; OCT: organic cation transporter.
To be a transdermal drug delivery, the skin permeability, which defines the rate of a chemical penetrating across the stratum corneum, will be checked to improve its efficacy. A molecule will perfectly penetrate the skin if log Kp was higher than −2.5 cm/h. All phytocompounds seem to possess moderate to good skin penetrability among them, those of scopoletin, sedanolide, (-)-Caryophyllene oxide and hordenine. The Caco-2 cell line is composed of human epithelial colorectal adenocarcinoma cells. Caco-2 permeability can predict the intake of oral drugs as Caco-2. All compounds displayed moderate to potent (log Papp values >0.90 cm/s) Caco-2 permeability values. Among them, scopoletin, sedanolide, (-)-Caryophyllene oxide and hordenine were predicted to have the strongest Caco-2 permeability.
P-gp is a key to estimate active efflux through biological membranes and known as the most important member among ATP-binding cassette transporters or ABC-transporters used to protect the central nervous system (CNS) from xenobiotics. None of the selected phytocompounds were P-gp inhibitor/substrate. Intestinal absorption was also assessed and most of the identified compounds were highly absorbed by the intestine. The skin permeation as given by LogKP was predicted and results demonstrated that identified compounds might be moderately or highly promoted to penetrate through the skin, which confirmed their drug-like properties. Many of them such as Sedanolide, and (-)-Caryophyllene oxide with log BB (logarithm value of brain to plasma concentration ratio) greater than 0.3 were subjected to have more potential to cross brain blood barrier (BBB). Only fewer compounds like arachidonic acid, hexadecanamide and hordenine have the ability to penetrate to CNS. (-)-Caryophyllene oxide and hordenine are amongst the phytoconstituents that will be more distributed with distribution volume logVDss of 0.564 L/kg and 0.887 L/kg in the tissues, respectively.
Human cytochrome P450 (CYP) isoforms involved in drug metabolism in liver were also generated. Amongst them, CYP3A4 is the major and most clinically relevant drug-metabolizing enzyme in the human body. Its inhibition could lead to drug toxicity, drug–drug interactions and other adverse effects. Some of them were non-inhibitors/substrate of any isoenzymes like sedanolide, however as a good result, more than 92% were found to be non-inhibitors of CYP3A4, isoenzyme responsible for the metabolism of about 60% of xenobiotics including drugs, carcinogens, steroids and eicosanoids.
To determine the excretion routes, the total clearance (CLTOT) for both hepatic and renal and renal organic cation transporter 2 (OCT2) substrate as expressed in log ml/min/kg were predicted. Results showed that about 98% of the identified phytocompounds exhibited a positive total clearance values and can be easily excreted.
The toxicity profile of all the identified phytoconstituents from S. repanda ethanolic crude extract has been predicted based on AMES toxicity, hepatotoxicity, hERG potassium channel inhibition and skin sensitization parameters. Results outlined that, out of the fifty four identified compounds, only three have a deviated mutagenic and hepatic toxicity potential, which means that about 95% are devoid of any risk of toxicity, 100% have no hERG I inhibition and 75% exerted no skin-sensitive effects.
To achieve more information for better bioavailability and drug-likeness of the identified compounds, we present an example of the top four suitable phytocompounds that can be delivered more as a potent candidate for drug discovery (Figure 7). The results of the bioavailability radar have been depicted by the lipophilicity: XLOGP3 between −0.7 and +5.0, size: MW between 150 and 500 g/mol, polarity: TPSA between 20 and 130 Å2, solubility: log S not higher than 6, saturation: fraction of carbons in the sp3 hybridization not less than 0.25 and flexibility: no more than 9 rotatable bonds with the colored zone defined the desired physicochemical space for good oral bioavailability indicating that they possess good drug-likeness properties.
Figure 7.
Top bioavailability radar of the phytocompounds based on physicochemical indices ideal for oral bioavailability.
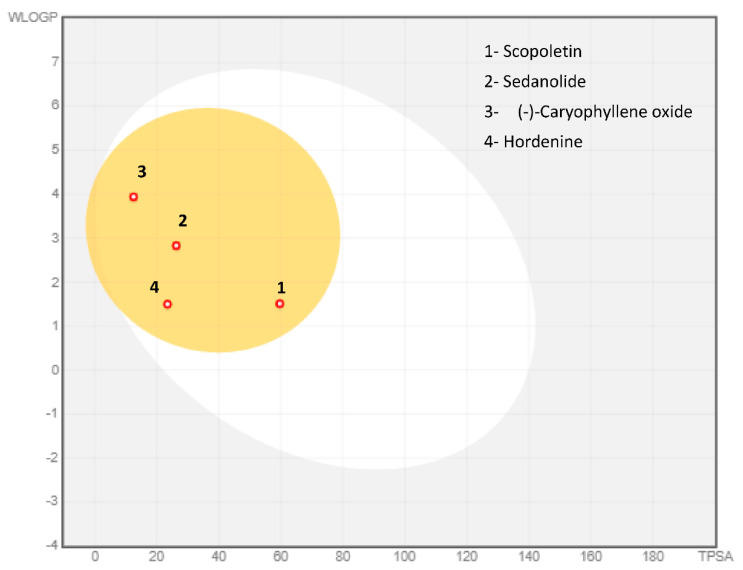
An example of the BOILED-Egg model (Brain or IntestinaL EstimateD permeation method) (Figure 8) prediction of gastrointestinal (GI) absorption and BBB permeation of the same selected compounds have been exploited. Results shows that the compounds appeared with a red point in the yellow ellipse having high probability of brain penetration and are non-substrate of P-gp (PGP-).
Figure 8.
BOILED-Egg model of the volatile constituents using the Swiss ADME predictor.
3. Materials and Methods
3.1. Plant Collection, Storage and Sequence Deposition
S. repanda whole plant was collected from the wild regions of Gujarat state, India during the period of July–August 2020. The plant was identified by the molecular sequencing method, plus by its taxonomic characters (Figure 9). The voucher specimen (BVBRC035) was deposited at bapalal vaidya botanical garden, department of biosciences, Veer Narmad South Gujarat University, Surat, Gujarat, India. rbcL gene nucleotide sequence (accession number MT795925) was deposited to NCBI. The whole plant was dried in an oven, followed by grinding into a fine powder and was then stored airtight containers. The percentage yield of the plant extract was calculated according to the following formula:
| (1) |
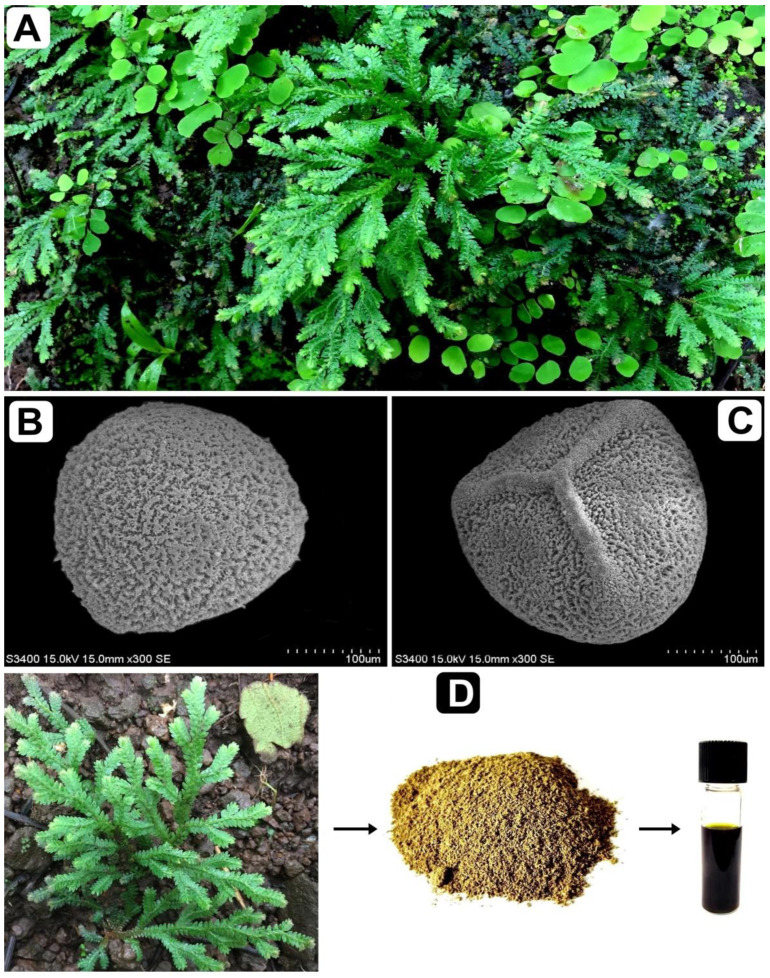
Figure 9.
S. repanda plant: (A) in the wild, (B,C) the structure of spores under scanning electron microscope (SEM) and (D) preparation of the crude extract.
3.2. S. repanda Crude Extraction
Ethanol (85%) was used for soaking of 20 g S. repanda powder for 24 h at 37 °C with vigorous shaking. Whatman no. 1 filter paper was used for filtering the ethanol phase mixture and then concentrated using a rotary evaporator to get the dried residue. Stock solution of crude ethanolic extract was then further used for performing various biological activities such as antibacterial, antioxidant and anticancer.
3.3. Antibacterial Assay
3.3.1. Bacterial Strains
Common pathogenic bacterial strains; S. flexneri (MTCC 1457), S. aureus (MTCC 96), E. coli (MTCC 9537) and P. aeruginosa (MTCC741) was used to carried out the antibacterial activity. They were obtained from the Microbial Type Culture Collection (MTCC), Chandigarh, India, and maintained on Muller–Hinton agar (MHA). To bring up to 0.5 McFarland standard 108 colony forming units/mL (CFU/mL), sterile saline solution was used and culture turbidity was adjusted.
3.3.2. Microdilution Method
Antibacterial activity of S. repanda crude extract was performed in 96-well microtiter plates against pathogenic bacteria, as described previously [83]. The inoculums were prepared from a 6 h Muller–Hinton broth (MHB) culture, and suspensions were adjusted to 0.5 McFarland turbidity standards (108 CFU/mL). S. repanda crude extract was diluted to twofold ranging from 1000 to 0.48 µg/mL (80 µL as final volume) with a DMSO concentration >1%. Afterward, 20 µL of bacterial suspensions and 100 µL of MHB were loaded onto microtiter plates. Plates were then incubated at 37 °C for 24 h. At the end of the incubation period, microtiter plates were read using a spectrophotometer at 620 nm. Chloramphenicol, a standard antibiotic, was used as a positive control. MHB + DMSO was used as a vehicle control, and MHB alone was used as a sterility control. MIC was recorded as the plant extract with the lowest concentration and has shown absolute inhibition of observable growth [84].
3.4. Antioxidant Assays
3.4.1. Determination of Free Radical Scavenging Effects of Antioxidants Using the DPPH Method
S. repanda crude extract antioxidant activity was measured against DPPH free radicals in relation to radical scavenging capability [85]. Crude extract of varied concentrations (100–500 μg/mL) were mixed in the tubes containing 2 mL of DPPH solution (6 × 10−5 M) in dimethyl sulfoxide (DMSO), followed by dark incubation for 1 h. After incubation, a decrease in absorbance was measured at 517 nm. As a standard, ascorbic acid was used; as a blank, DMSO was used; and as a control, DPPH solution without crude extract was used. Calculation of the percentage of scavenging of DPPH free radicals was then estimated as follows:
| DPPH scavenging activity (%) = (A0 − A1)/A0 × 100 | (2) |
whereas,
| A0 = absorbance of the control | (3a) |
| A1 = absorbance of the sample | (3b) |
3.4.2. Hydrogen Peroxide (H2O2) Scavenging Assay
H2O2 scavenging activity was measured as per the method used by Adnan et al. (2018) [19]. One milliliter of crude extract (100–500 μg/mL) was mixed with 1 mL of 2 mM H2O2 solution, prepared in 0.1 M phosphate buffer (pH 7.4). The tubes were then incubated for 10 min at room temperature, and absorbance was measured at 230 nm. Absorbance was determined against a blank solution (phosphate buffer without H2O2). Standard ascorbic acid was used as positive control. Calculation of the percentage of scavenging of H2O2 was then estimated as follows:
| Inhibition (%) = (A0 − A1)/A0) × 100 | (4) |
whereas,
| A0 = absorbance of the control | (5a) |
| A1 = absorbance of the extract/standard | (5b) |
3.5. Total Phenolic Content of S. repanda
The concentration of total phenolics in the extract was analyzed by the Folin–Ciocalteu colorimetric assay [86]. Firstly, crude extract (0.2 mL), deionized water (0.8 mL) and Folin–Ciocalteu reagent (0.1 mL) was incubated at room temperature for 3 min and then 0.3 mL of Na2CO3 (20% w/v) was added and the mixture was incubated at room temperature for 2 h. Absorbance of the mixture was read at 765 nm. A standard curve of gallic acid from 0 to 100 mg/L was prepared. Total phenolic content was expressed in mg gallic acid equivalents/g dry matter.
3.6. Cytotoxicity and Anticancer Assay (MTT Assay)
Cytotoxicity of S. repanda crude extract was performed against human normal colon cells (CRL-1831) and anticancer potential was performed against human lung (A549), breast (MCF-7) and colon (HCT-116) cancer cell lines. Cells were obtained from the NCCS, India. They were propagated in a humidified (5% CO2) atmosphere at 37 °C, and were maintained in 25 cm2 flask containing DMEM supplemented with 10% FBS. They were grown up to 80% confluence and were seeded in 96-well plates at a density of more than 1 × 105 cells per well with incubation conditions mentioned above. Cells were then stained with 0.4% Trypan Blue and viable cell numbers were calculated using a hemocytometer. However, assay was performed as triplicate wells for each concentration. Different concentration of S. repanda crude extract (100–500 μg/mL) was then used to treat the cells for 24 h. Followed by washing with PBS solution and subjected with 100 μL of MTT solution (3-(4,5-dimethylthiazolyl-2)-2,5 diphenyltetrazoliumbromide) (5 mg/mL), followed by incubation for 4 h. Finally, the medium was removed and 100 μL of DMSO was added to solubilize the formazan crystals. ELISA reader was then used to determine the amount of formazan crystal by measuring the absorbance at 570 nm. Fluorouracil (5FU) was used as a positive control. All assays were done in triplicate and 50% cytotoxic concentration (IC50) was calculated [5].
3.7. HR-LC–MS Analysis
Phytochemistry of S. repanda crude extract was analyzed using UHPLC-PDA-Detector Mass Spectrophotometer (HR-L=CMS 1290 Infinity UHPLC System), Agilent Technologies®, Santa Clara, CA, USA. The liquid chromatographic system consisted of the HiP sampler, binary gradient solvent pump, column compartment and quadrupole time of flight mass spectrometer (MS Q-TOF) with the dual Agilent Jet Stream Electrospray (AJS ES) ion source. Of the sample 10 µL was injected into the system, followed by separation in the SB-C18 column (2.1 mm × 50 mm, 1.8 µm particle size). Solvent A (1% formic acid in deionized water) and solvent B (acetonitrile) were used as solvents. Flow rate of 0.350 mL/min was used, while, MS detection was performed in MS Q-TOF. Compounds were identified via their mass spectra and their unique mass fragmentation patterns. Compound Discoverer 2.1, ChemSpider and PubChem were used as the main tools for the identification of the phytochemical constituents [5].
3.8. ADMET Analysis
Prediction of the pharmacokinetics and toxicity of the identified compounds from the S. repanda ethanolic crude extract was performed using the SwissADME (http://www.swissadme.ch/) and pkCSM (http://biosig.unimelb.edu.au/pkcsm/prediction) online tools [87,88,89].
3.9. Statistical Analysis
Results are expressed as mean ± SD of the number of experiments performed. A Student’s t-test for paired or unpaired values was performed and a p-value of <0.05 was considered statistically significant. Statistical analysis was conducted with software GraphPad Prism Version 7.03.
4. Conclusions
From this current work, it was concluded that S. repanda possessed strong antibacterial activity against different pathogenic bacterial strains of human interest, promised a source of antioxidant compounds and offered a worthy understanding about its anticancer potential. Moreover, S. repanda can also be used as a possible ingredient, nutraceutical or functional food, which requires further exploration, in vivo pharmacological and toxicological studies, to prove the unexplored beneficial aspects of S. repanda significant medicinal properties. In silico results indicated a good pharmacokinetic and safety profile of the phytochemical constituents, which make S. repanda ethanolic crude extract a potential drug candidate for the treatment of many diseases.
Author Contributions
Conceptualization, M.A., V.D.F., and M.P.; methodology, M.S. (Mejdi Snoussi), A.J., A.J.S., and S.A.A.; validation, M.S. (Manojkumar Sachidanandan), M.S. (Mejdi Snoussi), W.S.H., and A.M.A.; formal analysis, M.P., M.A., M.S. (Mejdi Snoussi), and A.J.S.; investigation, A.J., S.A.A. and A.M.A.; data curation, M.P., A.J., W.S.H., S.A.A. and M.S. (Manojkumar Sachidanandan); writing—original draft preparation, M.P. and M.A.; writing—review and editing, V.D.F., M.A., and M.S. (Mejdi Snoussi); visualization, M.P., and W.S.H.; supervision, M.A., and V.D.F.; project administration, M.P. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by Scientific Research Deanship at University of Ha’il-Saudi Arabia through project number RG-191194.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Sample of the extract is available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siddiqui A.J., Danciu C., Ashraf S.A., Moin A., Singh R., Alreshidi M., Patel M., Jahan S., Kumar S., Alkhinjar M.I.M., et al. Plants-Derived Biomolecules as Potent Antiviral Phytomedicines: New Insights on Ethnobotanical Evidences against Coronaviruses. Plants (Basel) 2020;9:1244. doi: 10.3390/plants9091244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mseddi K., Alimi F., Noumi E., Veettil V.N., Deshpande S., Adnan M., Hamdi A., Elkahoui S., Alghamdi A., Kadri A., et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020;13:6782–6801. doi: 10.1016/j.arabjc.2020.06.032. [DOI] [Google Scholar]
- 3.Adnan M., Patel M., Deshpande S., Alreshidi M., Siddiqui A.J., Reddy M.N., Emira N., De Feo V. Effect of Adiantum philippense Extract on Biofilm Formation, Adhesion With Its Antibacterial Activities Against Foodborne Pathogens, and Characterization of Bioactive Metabolites: An in vitro-in silico Approach. Front. Microbiol. 2020;11:823. doi: 10.3389/fmicb.2020.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noumi E., Snoussi M., Anouar E.H., Alreshidi M., Veettil V.N., Elkahoui S., Adnan M., Patel M., Kadri A., Aouadi K., et al. HR-LCMS-Based Metabolite Profiling, Antioxidant, and Anticancer Properties of Teucrium polium L. Methanolic Extract: Computational and In Vitro Study. Antioxidants. 2020;9:1089. doi: 10.3390/antiox9111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy M.N., Adnan M., Alreshidi M.M., Saeed M., Patel M. Evaluation of Anticancer, Antibacterial and Antioxidant Properties of a Medicinally Treasured Fern Tectaria coadunata with its Phytoconstituents Analysis by HR-LCMS. Anti Cancer Agents Med. Chem. 2020;20:1845–1856. doi: 10.2174/1871520620666200318101938. [DOI] [PubMed] [Google Scholar]
- 6.Alreshidi M., Noumi E., Bouslama L., Ceylan O., Veettil V.N., Adnan M., Danciu C., Elkahoui S., Badraoui R., Al-Motair K.A., et al. Phytochemical Screening, Antibacterial, Antifungal, Antiviral, Cytotoxic, and Anti-Quorum-Sensing Properties of Teucrium polium L. Aerial Parts Methanolic Extract. Plants. 2020;9:1418. doi: 10.3390/plants9111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin A., Manickam V.S. Medicinal pteridophytes from the Western Ghats. Nat. Prod. Repos. 2007;6:611–618. [Google Scholar]
- 8.Singh S., Singh R. A Review on Endemic Indian Resurrecting Herb Selaginella bryopteris (L.) Bak ‘Sanjeevani’. Int. J. Pharm. Sci. Res. 2015;6:50–56. [Google Scholar]
- 9.Han B.H., Chi H.J., Han Y.N., Ryu K.S. Screening on the anti-inflammatory activity of crud drugs. Korean J. Pharmacog. 1972;4:205–209. [Google Scholar]
- 10.Itokawa H., Mihashi S., Watanabe K., Natsumoto H., Hamanaka T. Studies on the constituents of crude drugs having inhibitory activity against contraction of the ileum caused by histamine or bariumchloride. Screening test for the activity of commercially availablecrude drugs and the related plant materials. Shoyakugaku Zasshi. 1983;37:223–228. [Google Scholar]
- 11.MacFoy C.A., Sama A.M. Medicinal plants in Pujehun District of Sierra Leone. J. Ethnopharmacol. 1983;8:215–223. doi: 10.1016/0378-8741(83)90055-7. [DOI] [PubMed] [Google Scholar]
- 12.Han D.S., Lee S.J., Lee H.K. Ethnobotanical survey in Korea; Proceedings of the Fifth Asian Symposium on Medicinal Plants and Spices; Seoul, Korea. 20–24 August 1984; p. 125. [Google Scholar]
- 13.Winkelman M. Frequently used medicinal plants in Baja California Norte. J. Ethnopharmacol. 1986;18:109–131. doi: 10.1016/0378-8741(86)90024-3. [DOI] [PubMed] [Google Scholar]
- 14.Darias V., Bravo L., Rabanal R., Sánchez Mateo C., González Luis R.M., Hernández Pérez A.M. New contribution to the ethnopharmacological study of the Canary Islands. J. Ethnopharmacol. 1989;25:77–92. doi: 10.1016/0378-8741(89)90047-0. [DOI] [PubMed] [Google Scholar]
- 15.Ono K., Nakane H., Meng Z.M., Ose Y., Sakai Y., Mizuno M. Differential inhibitory effects of various herb extracts on the activities of reverse transcriptase and various deoxyribonucleic acid (DNA) polymerases. Chem. Pharm. Bull. 1989;37:1810–1812. doi: 10.1248/cpb.37.1810. [DOI] [PubMed] [Google Scholar]
- 16.Meng Z.M., Saki Y., Ose Y., Sato T., Nagase H., Kito H., Sato M., Mizuno M., Ono K., Nakane H. Antimutagenic activity by the medicinal plants in traditional chinese medicines. Shoyakugaku Zasshi. 1990;44:225–229. [Google Scholar]
- 17.Lin R.C., Skaltsounis A.L., Seguin E., Tillequin F., Koch M. Phenolic Constituents of Selaginella doederleinii. Planta Med. 1994;60:168–170. doi: 10.1055/s-2006-959443. [DOI] [PubMed] [Google Scholar]
- 18.De Sá P.G., Nunes X.P., de Lima J.T., de Siqueira Filho J.A., Fontana A.P., Siqueira Jde S., Quintans-Júnior L.J., Damasceno P.K., Branco C.R., Branco A., et al. Antinociceptive effect of ethanolic extract of Selaginella convoluta in mice. BMC Complement. Altern. Med. 2012;12:187. doi: 10.1186/1472-6882-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adnan M., Patel M., Reddy M.N., Alshammari E. Formulation, evaluation and bioactive potential of Xylaria primorskensis terpenoid nanoparticles from its major compound xylaranic acid. Sci. Rep. 2018;8:1740. doi: 10.1038/s41598-018-20237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants (Basel) 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo Y.E., Song J., Bae S. Use of Plant and Herb Derived Medicine for Therapeutic Usage in Cardiology. Medicines (Basel) 2018;5:38. doi: 10.3390/medicines5020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malviya J., Joshi V., Singh K. Antimicrobial activity of some ethno-medicinal plants used by Baiga Tribes from Amarkantak, India. Adc. Life Sci. Technnol. 2012;4:19–26. [Google Scholar]
- 23.Upreti K., Jalal J.S., Tewari L., Joshi G., Tewari G. Ethnomedicinal uses of Pteridophytes of Kumaun Himalaya, Uttarakhand, India. J. Am. Sci. 2009;5:167–170. [Google Scholar]
- 24.Baskaran X.R., Geo Vigila A.V., Zhang S.Z., Feng S.X., Liao W.B. A review of the use of pteridophytes for treating human ailments. J. Zhejiang Univ. Sci. B. 2018;19:85–119. doi: 10.1631/jzus.B1600344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma M., Gangwar M., Sahai M., Nath G., Singh T.D. Antimicrobial Activity of Phytochemicals Isolated from Selaginella bryopteris. Chem. Nat. Compounds. 2015;51:341–345. doi: 10.1007/s10600-015-1277-9. [DOI] [Google Scholar]
- 26.Nallaiyan S., Doraiswamy H. Phytochemical Activity of Leaves of Selaginella involvens and Selaginella inaequalifolia extracts on Poultry Pathogens. Int J. Curr. Res. 2011;3:65–68. [Google Scholar]
- 27.Irudayaraj V.J.M., Johnson M., Selvan N. Preliminary phytochemical and antimicrobial studies on a spike-moss Selaginella inaequalifolia (Hook. &Grev.) Spring. Asian Pac. J. Trop. Med. 2010;3:957–960. [Google Scholar]
- 28.Macêdo L.A.R.D.O., Oliveira Júnior R.G.D., Souza G.R., de Oliveira A.P., de Lavor É.M., Silva M.G.E., Pacheco A.G.M., de Menezes I.R.A., Coutinho H.D.M., Pessoa C.D.Ó., et al. Chemical composition, antioxidant and antibacterial activities and evaluation of cytotoxicity of the fractions obtained from Selaginella convoluta (Arn.) Spring (Selaginellaceae) Biotechnol. Biotechnol. Equip. 2018;32:506–512. doi: 10.1080/13102818.2018.1431055. [DOI] [Google Scholar]
- 29.Choi S.M., Lee K.Y., Jang E.J., Cha S.M., Cha J.D. Antimicrobial activity of Selaginella tamariscina extract against oral bacteria. Dent. Oral. Craniofac. Res. 2019;5:1–7. [Google Scholar]
- 30.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sah N.K., Singh S.N., Sahdev S., Banerji S., Jha V., Khan Z., Hasnain S.E. Indian herb ‘Sanjeevani’ (Selaginella bryopteris) can promote growth and protect against heat shock and apoptotic activities of ultra violet and oxidative stress. J. Biosci. 2005;30:499–505. doi: 10.1007/BF02703724. [DOI] [PubMed] [Google Scholar]
- 32.Miao N., Tao H., Tong C., Xuan H., Zhamg G. The Selaginella tamariscina (Beauv.) Spring complex in the treatment of experimental diabetes and its effect on blood rheology. China J. Chin. Mater. Med. 1996;21:493–495, 512. [PubMed] [Google Scholar]
- 33.Li S., Zhu R., Zhong M., Zhang Y., Huang K., Zhi X., Fu S. Effects of ultrasonic-assistant extraction parameters on total flavones yield of Selaginella doederleinii and its antioxidant activity. J. Med. Plant. Res. 2010;4:1743–1750. [Google Scholar]
- 34.Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl. Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 35.Priscilla J.T., Geethaa S., Sreeramanan S., Ong M.T. Brine shrimp lethality test and anti-proliferation test against human cancer-origin cell lines using ethanolic and water extracts of selaginelladoederleinii hieron. J. Biomed. Pharm. Res. 2014;3:63–69. [Google Scholar]
- 36.Yang S.F., Chu S.C., Liu S.J., Chen Y.C., Chang Y.Z., Hsieh Y.S. Antimetastatic activities of Selaginella tamariscina (Beauv.) on lung cancer cells in vitro and in vivo. J. Ethnopharmacol. 2007;110:483–489. doi: 10.1016/j.jep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Lee I.S., Nishikawa A., Furukawa F., Kasahara K., Kim S.U. Effects of Selaginella tamariscina on in vitro tumor cell growth, p53 expression, G1 arrest and in vivo gastric cell proliferation. Cancer Lett. 1999;144:93–99. doi: 10.1016/S0304-3835(99)00202-5. [DOI] [PubMed] [Google Scholar]
- 38.Lee J.S., Lee M.S., Oh W.K., Sul J.Y. Fatty acid synthase inhibition by amentoflavone induces apoptosis and antiproliferation in human breast cancer cells. Biol. Pharm. Bull. 2009;32:1427–1432. doi: 10.1248/bpb.32.1427. [DOI] [PubMed] [Google Scholar]
- 39.Jing Y., Zhang G., Ma E., Zhang H., Guan J., He J. Amentoflavone and the extracts from Selaginella tamariscina and their anticancer activity. Chin. Herb Med. 2010;5:226–229. [Google Scholar]
- 40.Handayani S., Hermawan A., Meiyanto E., Udin Z. Induction of Apoptosis on MCF-7 cells by Selaginella Fractions. J. Appl. Pharm. Sci. 2013;3:31. [Google Scholar]
- 41.Li J., Lei X., Chen K.-L. Comparison of cytotoxic activities of extracts from Selaginella species. Pharmacogn. Mag. 2014;10:529. doi: 10.4103/0973-1296.141794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellati F., Benvenuti S., Magro L., Melegari M., Soragni F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J. Pharm. Biomed. Anal. 2004;35:289–301. doi: 10.1016/S0731-7085(03)00645-9. [DOI] [PubMed] [Google Scholar]
- 43.Lan L., Wang Y., Pan Z., Wang B., Yue Z., Jiang Z., Li L., Wang C., Tang H. Rhamnetin induces apoptosis in human breast cancer cells via the miR-34a/Notch-1 signaling pathway. Oncol. Lett. 2019;17:676–682. doi: 10.3892/ol.2018.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham I., Joshi R., Pardasani P., Pardasani R. Recent advances in 1,4-benzoquinone chemistry. J. Braz. Chem. Soc. 2011;22:385–421. doi: 10.1590/S0103-50532011000300002. [DOI] [Google Scholar]
- 45.Fidyt K., Fiedorowicz A., Strządała L., Szumny A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016;5:3007–3017. doi: 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pittillo R., Hunt D. Antibiotics. Springer; Berlin/Heidelberg, Germany: 1967. Azaserine and 6-diazo-5-oxo-l-norleucine (DON) pp. 481–493. [Google Scholar]
- 47.Król S.K., Kiełbus M., Rivero-Müller A., Stepulak A. Comprehensive review on betulin as a potent anticancer agent. BioMed Res. Int. 2015;2015:584189. doi: 10.1155/2015/584189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z., Hao W., Hu J., Mi X., Han Y., Ren S., Jiang S., Wang Y., Li X., Li W. Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-κB and PI3K/Akt Signal Pathways. Antioxidants. 2019;8:395. doi: 10.3390/antiox8090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donio M.B.S., Ronica F.A., Viji V.T., Velmurugan S., Jenifer J.S.C.A., Michaelbabu M., Dhar P., Citarasu T. Halomonas sp. BS4, A biosurfactant producing halophilic bacterium isolated from solar salt works in India and their biomedical importance. SpringerPlus. 2013;2:149. doi: 10.1186/2193-1801-2-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sari M., Chung Y., Agatha F., Kim H.K. Evaluation of antioxidant and antimicrobial activity of phenolic lipids produced by the transesterification of 4-hydroxyphenylacetic acid and triglycerides. Appl. Biol. Chem. 2019;62:5. doi: 10.1186/s13765-019-0419-3. [DOI] [Google Scholar]
- 51.Girennavar B., Cepeda M.L., Soni K.A., Vikram A., Jesudhasan P., Jayaprakasha G., Pillai S.D., Patil B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008;125:204–208. doi: 10.1016/j.ijfoodmicro.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 52.Zhao B., Tomoda Y., Mizukami H., Makino T. 9-Oxo-(10E, 12E)-octadecadienoic acid, a cytotoxic fatty acid ketodiene isolated from eggplant calyx, induces apoptosis in human ovarian cancer (HRA) cells. J. Nat. Med. 2015;69:296–302. doi: 10.1007/s11418-015-0892-x. [DOI] [PubMed] [Google Scholar]
- 53.Yi E.-Y., Kim Y.-J. Betaine inhibits in vitro and in vivo angiogenesis through suppression of the NF-κB and Akt signaling pathways. Int. J. Oncol. 2012;41:1879–1885. doi: 10.3892/ijo.2012.1616. [DOI] [PubMed] [Google Scholar]
- 54.Liu J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005;100:92–94. doi: 10.1016/j.jep.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 55.Kashyap D., Tuli H.S., Sharma A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016;146:201–213. doi: 10.1016/j.lfs.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Xiao F., Wang C., Yin H., Yu J., Chen S., Fang J., Guo F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget. 2016;7:63679. doi: 10.18632/oncotarget.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baser K., Kirimer N., Tümen G. Pulegone-rich essential oils of Turkey. J. Essent. Oil Res. 1998;10:1–8. doi: 10.1080/10412905.1998.9700830. [DOI] [Google Scholar]
- 58.Tabana Y.M., Hassan L.E.A., Ahamed M.B.K., Dahham S.S., Iqbal M.A., Saeed M.A., Khan M.S.S., Sandai D., Majid A.S.A., Oon C.E. Scopoletin, an active principle of tree tobacco (Nicotiana glauca) inhibits human tumor vascularization in xenograft models and modulates ERK1, VEGF-A, and FGF-2 in computer model. Microvasc. Res. 2016;107:17–33. doi: 10.1016/j.mvr.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Ou S., Kwok K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004;84:1261–1269. doi: 10.1002/jsfa.1873. [DOI] [Google Scholar]
- 60.Chen Y.-Y., Lee M.-H., Hsu C.-C., Wei C.-L., Tsai Y.-C. Methyl cinnamate inhibits adipocyte differentiation via activation of the CaMKK2–AMPK pathway in 3T3-L1 preadipocytes. J. Agric. Food Chem. 2012;60:955–963. doi: 10.1021/jf203981x. [DOI] [PubMed] [Google Scholar]
- 61.Zheng L., Yan X., Han X., Chen H., Lin W., Lee F.S., Wang X. Identification of norharman as the cytotoxic compound produced by the sponge (Hymeniacidon perleve)-associated marine bacterium Pseudoalteromonas piscicida and its apoptotic effect on cancer cells. Biotechnol. Appl. Biochem. 2006;44:135–142. doi: 10.1042/BA20050176. [DOI] [PubMed] [Google Scholar]
- 62.Sıdır İ., Sıdır Y.G. Investigation on the interactions of E-4-methoxycinnamic acid with solvent: Solvatochromism, electric dipole moment and pH effect. J. Mol. Liq. 2018;249:1161–1171. doi: 10.1016/j.molliq.2017.11.136. [DOI] [Google Scholar]
- 63.Liu X., Jiang Q., Liu H., Luo S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019;52:7. doi: 10.1186/s40659-019-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang Y., Lou Z., Yang L., Wang H. Screening of antimicrobial compounds against Salmonellaty phimurium from burdock (Arctium lappa) leaf based on metabolomics. Eur. Food Res. Technol. 2015;240:1203–1209. doi: 10.1007/s00217-015-2423-0. [DOI] [Google Scholar]
- 65.Panat N.A., Amrute B.K., Bhattu S., Haram S.K., Sharma G.K., Ghaskadbi S.S. Antioxidant profiling of C3 quercetin glycosides: Quercitrin, Quercetin 3-β-d-glucoside and Quercetin 3-O-(6”-O-malonyl)-β-d-glucoside in cell free environment. Free Rad. Antiox. 2015;5:90–100. [Google Scholar]
- 66.Hsieh S.-L., Chen C.-T., Wang J.-J., Kuo Y.-H., Li C.-C., Hsieh L.-C., Wu C.-C. Sedanolide induces autophagy through the PI3K, p53 and NF-κB signaling pathways in human liver cancer cells. Int. J. Oncol. 2015;47:2240–2246. doi: 10.3892/ijo.2015.3206. [DOI] [PubMed] [Google Scholar]
- 67.Kim H., Kong H., Choi B., Yang Y., Kim Y., Lim M.J., Neckers L., Jung Y. Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm. Res. 2005;22:1499–1509. doi: 10.1007/s11095-005-6250-z. [DOI] [PubMed] [Google Scholar]
- 68.Sato Y., Itagaki S., Kurokawa T., Ogura J., Kobayashi M., Hirano T., Sugawara M., Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011;403:136–138. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 69.Sisler H.D., Siegel M.R. Mechanism of Action. Springer; Berlin/Heidelberg, Germany: 1967. Cycloheximide and other glutarimide antibiotics; pp. 283–307. [Google Scholar]
- 70.Yin J., Li X., Huang F., Lu M., Yang J., Zhu L. Chemical composition, antioxidant and anticancer activity of the essential oil from myric rubra leaves; Proceedings of the 5th International Conference on Agricultural and Biological Sciences (ABS); Macau, China. 21–24 July 2019; p. 012085. [Google Scholar]
- 71.Seo G.Y., Lim Y., Koh D., Huh J.S., Hyun C., Kim Y.M., Cho M. TMF and glycitin act synergistically on keratinocytes and fibroblasts to promote wound healing and anti-scarring activity. Exp. Mol. Med. 2017;49:e302. doi: 10.1038/emm.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan X., Qi M., Li P., Zhan Y., Shao H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7:50. doi: 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blanford H.F. A list of the ferns of Shimla in the NW. Himalaya between Levels of 4500 and 10,5000 feet. J. Asiatic Soc. Bengal. 1888;57:294–315. [Google Scholar]
- 74.Jiang D., Rasul A., Batool R., Sarfraz I., Hussain G., Mateen Tahir M., Qin T., Selamoglu Z., Ali M., Li J. Potential anticancer properties and mechanisms of action of formononetin. BioMed Res. Int. 2019;2019:5854315. doi: 10.1155/2019/5854315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maharaj D.S., Glass B.D., Daya S. Melatonin: New places in therapy. Biosci. Rep. 2007;27:299–320. doi: 10.1007/s10540-007-9052-1. [DOI] [PubMed] [Google Scholar]
- 76.Morel I., Lescoat G., Cogrel P., Sergent O., Pasdeloup N., Brissot P., Cillard P., Cillard J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993;45:13–19. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q., Bao J., Yang J. Genistein-triggered anticancer activity against liver cancer cell line HepG2 involves ROS generation, mitochondrial apoptosis, G2/M cell cycle arrest and inhibition of cell migration. Arch. Med. Sci. AMS. 2019;15:1001. doi: 10.5114/aoms.2018.78742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chouinard F., Turcotte C., Guan X., Larose M.C., Poirier S., Bouchard L., Provost V., Flamand L., Grandvaux N., Flamand N. 2-Arachidonoyl-glycerol- and arachidonic acid-stimulated neutrophils release antimicrobial effectors against E. coli, S. aureus, HSV-1, and RSV. J. Leukoc. Biol. 2013;93:267–276. doi: 10.1189/jlb.0412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arentsen H.C., Jansen C.F., Hulsbergen-van de Kaa C.A., Laihia J.K., Pylkkänen L., Leino L., Oosterwijk E., Witjes J.A. Antitumor effects of cis-urocanic acid on experimental urothelial cell carcinoma of the bladder. J. Urol. 2012;187:1445–1449. doi: 10.1016/j.juro.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 80.Dutta S., Ray S., Nagarajan K. Glutamic acid as anticancer agent: An overview. Saudi. Pharm. J. SPJ. 2013;21:337–343. doi: 10.1016/j.jsps.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imran M., Rauf A., Shah Z.A., Saeed F., Imran A., Arshad M.U., Ahmad B., Bawazeer S., Atif M., Peters D.G., et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019;33:263–275. doi: 10.1002/ptr.6227. [DOI] [PubMed] [Google Scholar]
- 82.Cho E., Chung E.Y., Jang H.Y., Hong O.Y., Chae H.S., Jeong Y.J., Kim S.Y., Kim B.S., Yoo D.J., Kim J.S., et al. Anti-cancer Effect of Cyanidin-3-glucoside from Mulberry via Caspase-3 Cleavage and DNA Fragmentation in vitro and in vivo. Anti Cancer Agents Med. Chem. 2017;17:1519–1525. doi: 10.2174/1871520617666170327152026. [DOI] [PubMed] [Google Scholar]
- 83.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2014. 24th Informational Supplement. [Google Scholar]
- 84.Srinivasan S., Beema Shafreen R.M., Nithyanand P., Manisankar P., Pandian S.K. Synthesis and in vitro antimicrobial evaluation of novel fluoroquinolone derivatives. Eur. J. Med. Chem. 2010;45:6101–6105. doi: 10.1016/j.ejmech.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 85.Adnan M., Patel M., Hadi S. Functional and health promoting inherent attributes of Enterococcus hirae F2 as a novel probiotic isolated from the digestive tract of the freshwater fish Catla catla. PeerJ. 2017;5:e3085. doi: 10.7717/peerj.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waterhouse A.L. Determination of total phenolics. In: Wrolstad E.R.E., editor. Current Protocols in Food Analytical Chemistry. John Wiley and Sons, Inc.; New York, NY, USA: 2001. pp. L1.1.1.–L1.1.8. [Google Scholar]
- 87.Kadri A., Aouadi K. In vitro antimicrobial and α-glucosidase inhibitory potential of enantiopure cycloalkylglycine derivatives: Insights into their in silico pharmacokinetic, druglikeness, and medicinal chemistry properties. J. Appl. Pharm. Sci. 2020;10:107–115. [Google Scholar]
- 88.Othman I.M., Gad-Elkareem M.A., Aouadi K., Kadri A., Snoussi M. Design, synthesis ADMET and molecular docking of new imidazo [4,5-b] pyridine-5-thione derivatives as potential tyrosyl-tRNA synthetase inhibitors. Bioorganic. Chem. 2020;102:104105. doi: 10.1016/j.bioorg.2020.104105. [DOI] [PubMed] [Google Scholar]
- 89.Ghannay S., Kadri A., Aouadi K. Synthesis, in vitro antimicrobial assessment, and computational investigation of pharmacokinetic and bioactivity properties of novel trifluoromethylated compounds using in silico ADME and toxicity prediction tools. Mon. Chem. Chem. Mon. 2020;151:267–280. doi: 10.1007/s00706-020-02550-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.