Fig. 9.
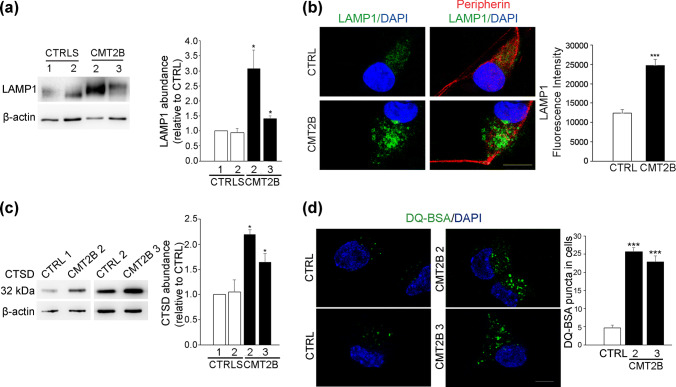
Lysosomal activity in iPSC-derived sensory neurons. a Lysates of neurons derived from controls and CMT2B fibroblasts carrying the RAB7V162M mutation (patient 2 and 3) were subjected to immunoblotting using anti-LAMP1 and anti-β-actin antibodies. Intensities of bands were measured by densitometry and normalized against β-actin. Data represent the mean ± SEM of three experiments. Statistical analysis was performed using Student’s t test with control neurons as referring sample. *p < 0.05. b Control and CMT2B neurons from patient 2 were fixed and immunolabeled with anti-LAMP1 and anti-peripherin antibodies followed by Alexa488- and Alexa568-conjugated secondary antibody respectively while nuclei were stained with DAPI. Bars 10 µm. LAMP1 intensities were measured using ImageJ and Corrected Total Cell Fluorescence (CTCF) was calculated. Data represent the mean ± SEM of at least 50 cells analyzed. Student’s t test was used for statistical analysis. ***p < 0.001. c Lysates of neurons derived from controls and CMT2B fibroblasts (patient 2 and 3) were subjected to immunoblotting using anti-cathepsin D and anti-β-actin antibodies. Intensities of bands were measured by densitometry and normalized against β-actin. Data represent the mean ± SEM of three experiments. Statistical analysis was performed using Student’s t test with control neurons as referring sample. *p < 0.05. d Control and CMT2B neurons from patient 2 and 3 were treated with DQ-BSA for 24 h, then fixed, labeled with DAPI and observed on a confocal microscope. Bars 10 µm. DQ-BSA puncta in cells were measured using ImageJ. Data represent the mean ± SEM of at least 50 cells analyzed. For DQ-BSA quantification, Student’s t test was used for statistical analysis. ***p < 0.001