Abstract
Background:
Interleukin-27 mRNA is highly enriched in the tissue of hepatocellular carcinoma. Overexpression of interleukin-27 gene has been found to increase T cell expression of inhibitory receptors, an immunosuppressive feature in tumor microenvironment, that promotes the development of hepatocellular carcinoma.
Methods:
Two parallel case-control studies of hepatocellular carcinoma, each with 100 case-control pairs were conducted in the Singapore Chinese Health Study and the Shanghai Cohort Study to examine the association between serum interleukin-27 levels and risk of developing hepatocellular carcinoma. The interleukin-27 concentrations were significantly elevated in sera collected from study participants 4–5 years prior to the diagnosis of hepatocellular carcinoma in both cohort studies.
Results:
Compared with the lowest tertile of interleukin-27, odds ratios (ORs)of hepatocellular carcinoma for the highest tertile of interleukin-27 was 46.08 [95% confidence interval (CI): 4.68–453.86] in the Singapore Chinese Health Study and 19.09 (95% CI: 3.81–95.57) in the Shanghai Cohort Study (both ptrend <0.001). The corresponding ORs in both cohort studies were 42.47 (95% CI: 8.30–217.40) among individuals negative for hepatitis B surface antigen (HBsAg) and 242.46 (95% IC: 38.42–1529.01) among those positive for HBsAg compared with the lowest tertile of interleukin-27 and negative HBsAg.
Conclusion:
Levels of interleukin-27 in prediagnostic sera were significantly associated with increased risk of hepatocellular carcinoma development.
Impact:
Interleukin-27 through its immunosuppressive property may play a significant role in the development of hepatocellular carcinoma. Serum levels of interleukin-27 may be used as a biomarker for prediction of hepatocellular carcinoma development.
Keywords: Cytokines, immunosuppression
INTRODUCTION
Liver cancer is the sixth most common cancer worldwide (13.9 cases per 100,00 men and 4.9 cases per 100,000 women) and the fourth most common cause of cancer death (12.7 cases per 100,000 men and 4.6 cases per 100,000 women) worldwide in 2018 (1). Hepatocellular carcinoma (HCC) accounts for 85–90% of primary liver cancers (2). Major risk factors for HCC are chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), heavy alcohol use, nonalcoholic fatty liver disease (NAFLD), and dietary exposure to aflatoxin B1 in certain regions (2–4). Globally, approximately 257 million persons are chronically infected with HBV and 71 million persons with HCV in 2017 (5). With increasing prevalence of obesity and diabetes globally, NAFLD is the most common liver disease, with a worldwide prevalence of 25% (6). However, it is estimated that only 1–3% of persons with viral hepatitis or NAFLD would eventually develop HCC over up to 30 years (2, 7). It is not clear what factors determine such a small proportion of population with these risk factors to progress and ultimately develop HCC.
Inflammation plays an important role in the development of HCC. Liver is the organ that is constantly exposed to a wide variety of immunomodulators from the intestine via portal vein (8). To ensure upkeep of local and systemic immune tolerance to foreign antigen, the liver develops an efficient adaptive immune mechanism (9). Interleukin (IL)-27 has recently been recognized as having anti-tumor and protumor properties (10). IL-27 is a heterodimeric member of the IL-12/IL-23/IL-35 cytokine family. IL-27 is mainly produced by cells of myeloid origin such as dendritic cells, macrophages and monocytes, in response to a variety of microbial and immune stimuli acting through Toll-like receptors (11–13). The liver contains a large population of these myeloid immune cells (14, 15). IL-27 can promote the growth and survival of Treg cells (16, 17), which exert immunosuppressive effects. Recent experimental studies have shown that overexpression of IL-27 gene increases T cell expression of inhibitory receptors including programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), lymphocyte-activating gene 3 (LAG3), and T-cell immunoglobulin and mucin-domain containing-3 (TIM3), and limits intensity and duration of T cell response to infection and cancer (18, 19). IL-27 mRNA is highly enriched in HCC, which has more than 100 times the average IL-27 mRNA of all other cancer types (Supplementary Figure S1) (www.cbioportal.org). These data suggest that IL-27 may play an important immunoregulatory role in the tumor microenvironment that promotes the initiation and progression of HCC.
Utilizing the resources of two ongoing population-based prospective cohort studies, we prospectively assessed the associations between serum IL-27 and the risk of developing HCC. We simultaneously conducted two parallel studies, with one serving as the discovery study and the other as the validation study, in these two cohort studies.
MATERIALS AND METHODS
Study Population
Subjects were drawn from two population-based cohort studies–the Shanghai Cohort Study and the Singapore Chinese Health Study (20, 21). These two cohort studies have been approved by the Institutional Review Boards of the Shanghai Cancer Institute, the National University of Singapore, and the University of Pittsburgh. The present study was approved by the Institutional Review Board of the University of Pittsburgh.
The Singapore Chinese Health Study enrolled 63,257 Chinese men and women, aged 45–74 years, in Singapore, from April 1993 to December 1998 (21). An in-person interview was administered by a trained interviewer to all study participants. A structured questionnaire was used to collect participant’s information on age, sex, dialect group, highest level of education attained, use of cigarette and other tobacco products, physical activity in the past year, occupational exposure, medical history and family history of cancer; for women, additional questions related to menstrual and reproductive histories were asked. In addition, a validated semi-quantitative food frequency questionnaire was used to collection participant’s information on habitual dietary intake including consumption of alcoholic beverages during the past 12 months (22). We requested non-fasting blood sample (20 mL) and single-void urine sample from randomly selected 3% participants during April 1994—December 1999, and all surviving participants beginning in January 2000. Overall, blood and/or urine samples were obtained from 32,535 participants, which was approximately 60% of eligible participants by the end of biospecimen collection phase on 30 April 2005. All collected samples were stored at −80°C until analysis.
The Shanghai Cohort Study was initiated in 1986. A total of 18,244 male residents aged 45–64 years in Shanghai, China, were enrolled from January 1986 to September 1989 (20, 23). An in-person interview was administered by a trained interviewer using a structured questionnaire for participant’s information on lifetime use of cigarettes and other tobacco products, consumption of alcoholic beverages, usual dietary habits, and medical history. In addition, a retired nurse collected a single 10-ml non-fasting blood sample and a single-void urine sample at the end of the interview from all participants. Serum was separated from whole blood within 4 hours and urine samples were processed within 10 hours and all specimens were stored at −72°C until analysis.
Case-Control Studies
All study participants were annually followed up for the incidence of cancer and death. For the Singapore Chinese Health Study, cancer cases were identified via linkage analyses with the databases of the nationwide Singapore Cancer Registry and deaths were ascertained in the Birth and Death Registry. The International Classification of Diseases-Oncology 10th edition code C22 defined HCC. For the Shanghai Cohort Study, cancer cases and deaths were identified through annual follow-up interviews to all surviving study participants or next of kin for those deceased. In addition, the record linkage analyses with the databases of the population-based Shanghai Cancer Registry and the Shanghai Municipal Vital Statistics Office provided additional information to supplement and/verify the diagnosis of cancer or causes of death. The follow-ups for cancer incidence and death of both cohort studies were virtually complete. To date, 56 participants (<0.1%) of the Singapore Chinese Health Study and 612 participants (3.4%) of the Shanghai Cohort Study had been cumulatively lost to follow-up.
As of 31 December 2015, we identified 216 incident HCC cases among participants of the Singapore Chinese Health Study who provided a pre-diagnostic serum sample. For the present study, we chose the first 100 incident HCC cases. We randomly selected one control subject per case among all potentially eligible subjects with available baseline serum samples. The control must be alive and free of cancer at the time of cancer diagnosis for the index case and was individually matched to the index case by age at enrollment (±3 years), gender, dialect group (Hokkien, Cantonese), date of biospecimen collection (±6 months), and date of baseline interview (±2 year).
In the Shanghai Cohort Study, we identified 402 incident HCC cases by 31 December 2015. For the validation study, we also chose first 100 incident HCC cases. Similarly, we randomly chose one control subject for each case among the cohort study participants who were free of cancer and alive during the time from blood draw to cancer diagnosis of the index case. The control was individually matched to the index case by age (± 2 years), date of blood draw (± 1 month), and the same neighborhood of residence at study enrollment. The present study included two parallel case-control studies of HCC from both the Singapore and Shanghai cohort studies.
Measurement of Serum Cytokine and Other Biomarkers
Serum samples from 400 study subjects were retrieved from the biorepositories of the Singapore Chinese Health Study and the Shanghai Cohort Study, respectively. All serum samples were stored at −72°C to −80°C until use with no more than three freeze-thaw cycles on ice. To ensure the comparability and reduce bias, the case or control status of the test samples was blind to laboratory technicians. The pair of the serum samples for each matched case-control set were tested in the same assay batch. The Millipore human Th17 Mag 17plex (catalog # HTH17MAG-14k-17) were designed to measure 17 cytokines: IL-1β, IL-9, IL-10, IL-12(p70), IL-15, IL-17A/CTLAB, IL-17E/IL-25, IL-17F, IL-21, IL-22, IL-23, IL-27, IL-28A/IFNλ2, IL-31, IL-33/NF-HEV, MIP-3α/CCL20, and TNFβ/Lymphotoxin α. The serum concentrations of these biomarkers were measured using the Luminex bead-based immunoassay and the fluorescence intensities were measured using the LabMAG™ system (Luminex Corporation, Austin TX). All except IL-27 had undetectable levels of the analytes on most of serum samples. We used commercial ELISA kits (Thermo Fisher Scientific, Pittsburgh, PA) to quantify sRAGE and IL-6 in all study samples. Serum HMGB1 was measured using the Shinotest/IBL/Tecan (Hamburg, Germany) ELISA.
The assays used for testing serum HBsAg and antibodies to HCV (anti-HCV) were described previously (24, 25). We tested all serum samples for the presence of HBsAg using a standard radioimmunoassay (AUSRIA, Abbott Laboratories, North Chicago, IL, USA), and anti-HCV using the ELISA version 2.0 kit (Ortho Diagnostic Systems, Raritan, NJ, USA), with confirmation of positive samples using the RIBA version 2.0 (Chiron, Emeryville, CA, USA). Serum anti-HCV was tested on the first 76 HCC cases and their matched controls of the Shanghai Cohort Study and the first 92 HCC cases and their matched controls of the Singapore Chinese Health Study, and stopped these tests thereafter due to extremely low prevalence of anti-HCV in both study populations (24, 25).
Statistical analysis
The distributions of serum IL-27, IL-6, HMGB1 and sRAGE were rightward skewed. Logarithmically transformed values were used in formal statistical testing, and geometric means and 95% confidence intervals (CIs) are presented. Body mass index (BMI) was categorized into <18.5 kg/m2 (underweight), 18.5–22.9 (normal weight), 23.0-<27.5 (overweight), and ≥27.5 (obesity) according to the criteria for Asian populations recommended by the World Health Organization (26). The alcohol consumption levels were classified as nondrinkers, moderate drinkers (≤21 drinks/week for men and ≤14 drinks/week for women) and heavy drinkers (>21 drinks/week for men and >14 drinks/week for women). We used χ2 (for categorical variables) or t-test (for continuous variables) statistics to examine the difference in the distributions of selected baseline demographic and lifestyle variables between HCC cases and controls. The analysis of covariance (ANCOVA) method was used to examine the difference in the log-values of serum IL-27, IL-6, HMGB1 and sRAGE among cases and control subjects, respectively, across different categories of covariates such as gender, BMI, smoking status (never, former, and current smokers), alcohol consumption level, and HBsAg serological status in the Shanghai and Singapore cohort studies separately. We used the extended ANCOVA method to compare the differences in log-values of serum IL-27, IL-6, HMGB1 and sRAGE between HCC cases and control subjects, which retained the case-control pairs with additionally adjusted variables such as BMI, alcohol consumption, cigarette smoking, and HBsAg serological status . Median values of IL-27 mRNA were calculated and Wilcoxon test was used to test the difference between tumor types.
We analyzed the data using the conditional logistic regression method that would retain the original matched case-control pairs for both studies to assess the associations for serum levels of IL-27, IL-6, HMGB1 and sRAGE with HCC risk. Study subjects were divided into tertiles according to the distribution of each of the biomarkers among control subjects within each of the two cohort studies (see the cutoff values in Supplementary Table S1). The magnitude for the association between levels of serum biomarkers and HCC risk was evaluated using odds ratios (ORs) and their 95% CIs. Ordinal values of tertile (i.e., 1, 2, and 3) were used for linear trend test for the levels of serum biomarkers and risk of HCC. The multivariate logistic regression model was used to adjust for potential confounding effect by the following covariables: cigarette smoking (never, former, and current smokers), alcohol consumption (nondrinkers, moderate drinkers, and heavy drinkers), BMI (<18.5, 18-<23, 23-<27.5, ≥27.5 kg/m2), and HBsAg or anti-HCV serological status (negative, positive).
Statistical analyses were carried out using SAS software version 9.4 (SAS Institute, Cary, NC). All p values reported are two-sided. The p values of less than 0.05 were considered to be statistically significant.
RESULTS
The mean age (± standard deviation) of cases at diagnosis of HCC was 70 (±7.6) years in the Singapore Chinese Health Study and 63 (±4.8) years in the Shanghai Cohort Study. The average time interval between blood collection and HCC diagnosis in the Singapore cohort Study and the Shanghai Cohort Study were 4.3 (± 2.3) years and 4.6 (±2.9) years, respectively.
Table 1 shows the distributions of selected baseline characteristics and risk factors for HCC in cases and control subjects in both cohort studies. Individuals who developed HCC had significantly higher prevalence of positive HBsAg at baseline in both cohort studies (41% in the Singapore cohort study and 53% in the Shanghai cohort study) than their respective controls (9% and 11%) whereas the prevalence of anti-HCV seropositivity was low (<2%) in both cohort studies. The prevalence of type 2 diabetes was significantly higher in participants who developed HCC than those who remained free of cancer in the Singapore cohort study whereas the corresponding figure in the Shanghai cohort study was extremely low, which was expected in China in the mid-1980s. Age, BMI, level of education, smoking and alcohol consumption were comparable between the case and control groups within each of the two cohort studies.
Table 1.
Baseline demographic and lifestyle characteristics of study participants who developed hepatocellular carcinoma (cases) and those who remained cancer free (controls), The Singapore Chinse Health Study and The Shanghai Cohort Study
| Characteristic | Singapore Cohort Study | Shanghai Cohort Study | |||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | Pa | Cases | Controls | Pa | ||
| Number of subjects | 100 | 100 | 100 | 100 | |||
| Age (year), mean ± SD | 66.4 ± 7.1 | 66.3 ± 6.9 | 0.936 | 58.5 ± 4.3 | 58.5 ± 4.2 | 0.941 | |
| Body mass index (kg/m2) | 24.2 ± 3.8 | 23.8 ± 3.5 | 0.461 | 22.2 ± 3.6 | 22.4 ± 3.1 | 0.787 | |
| Level of body mass index, % | |||||||
| <18.5 (kg/m2) | 3 | 3 | 0.871 | 10 | 8 | 0.433 | |
| 18.5 – <23.0 | 43 | 43 | 51 | 59 | |||
| 23.0 – <27.5 | 36 | 40 | 30 | 29 | |||
| ≥27.5 | 18 | 14 | 9 | 4 | |||
| Highest level of education, % | 0.125 | 0.954 | |||||
| No formal education | 22 | 12 | 7 | 6 | |||
| Primary school | 49 | 50 | 29 | 30 | |||
| Secondary school or above | 29 | 38 | 64 | 64 | |||
| Cigarette smoking, % | 0.553 | 0.583 | |||||
| Never smokers | 44 | 49 | 39 | 46 | |||
| Former smokers | 33 | 34 | 9 | 7 | |||
| Current smokers | 23 | 17 | 52 | 47 | |||
| Alcohol drinking, % | 0.209 | 0.714 | |||||
| Nondrinkers | 75 | 79 | 62 | 63 | |||
| Moderate drinkers | 22 | 21 | 26 | 22 | |||
| Heavy drinkersb | 3 | 0 | 12 | 15 | |||
| History of type 2 diabetes, % | 0.002 | 0.561 | |||||
| No | 70 | 88 | 99 | 98 | |||
| Yes | 30 | 17 | 1 | 2 | |||
| HBsAg serology, % | <0.001 | <0.001 | |||||
| Negative | 59 | 91 | 47 | 89 | |||
| Positive | 41 | 9 | 53 | 11 | |||
| Anti-HCV serology %c | |||||||
| Negative | 98.3 | 98.3 | 1.000 | 100 | 98.1 | 1.000 | |
| Positive | 1.7 | 1.7 | 0 | 1.9 | |||
2-sided P values were based on t test for continuous variables or chi-square test for categorical variables;
Heavy drinking was defined as >3 drinks/day for men and >2 drinks/day for women, and lower levels as moderate drinking;
The mean concentration of serum IL-27 in HCC cases was significantly higher (2.36 ng/ml) than that observed in controls (1.77 ng/ml) of the Singapore cohort study whereas the corresponding figures in the Shanghai cohort study were 2.59 (ng/ml) and 1.94 (ng/ml) (both p’s < 0.001) (Table 2). The differences in IL-27 levels between two cohort studies were not statistically significant in either controls (p = 0.122) or cases (p = 0.071). No statistically significant difference in concentrations of serum IL-6, HMGB1 and sRAGE between HCC cases and controls was found in either or both cohort studies combined (Table 2).
Table 2.
Serum concentrations of IL-27, HMGB1 and sRAGE in patients with hepatocellular carcinoma and control subjects in the Singapore Chinese Health Study and the Shanghai Cohort Study separately and combined
| Singapore Cohort Study | Shanghai Cohort Study | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | P | Cases | Controls | Pa | |||
| N | 100 | 100 | 100 | 100 | ||||
| Geometric mean (95% CI)a | ||||||||
| IL-27 (ng/ml) | 2.38 (2.20–2.58) | 1.76 (1.62–1.90) | <0.001 | 2.70 (2.40–3.02) | 1.88 (1.68–2.10) | <0.001 | ||
| IL-6 (pg/ml) | 2.20 (1.90–2.54) | 1.98 (1.70–2.30) | 0.358 | 4.12 (2.98–5.70) | 5.48 (3.96–7.58) | 0.273 | ||
| HMGB1 (ng/ml) | 1.36 (1.12–1.62) | 1.38 (1.16–1.66) | 0.896 | 2.58 (2.26–2.96) | 3.08 (2.70–3.52) | 0.104 | ||
| sRAGE (pg/ml) | 86.8 (77.1–97.7) | 91.1 (80.1–102.7) | 0.600 | 270.4 (227.3–321.8) | 275.9 (231.9–328.2) | 0.885 | ||
All geometric means and p values were derived from extended ANCOVA adjusted for matching variables and body mass index (<18.5, 18-<23, 23-<27.5, ≥27.5 kg/m2), alcohol consumption (nondrinkers, moderate drinkers, and heavy drinkers), cigarette smoking (never, former, current smokers), and serological status of hepatitis B surface antigen or antibodies to hepatitis C virus (negative, positive).
A strong association between higher serum levels of IL-27 and the risk of HCC was initially observed in the discovery cohort study and validated in the validation cohort study. In the Singapore cohort study, individuals with the second and third tertiles of baseline serum IL-27 were 8.74 (95% CI: 1.03–74.06) and 46.08 (95% CI: 4.68–453.86) times the risk of developing HCC, respectively, compared to the lowest tertile after adjustment for BMI, alcohol consumption levels, smoking status and HBsAg status (ptrend < 0.001) (Table 3). We repeated the same analysis in the Shanghai Cohort Study and validated this strong positive association. The ORs of HCC for the second and third tertile of IL-27 were 5.42 (95% CI: 1.33–22.07) and 19.09 (95% CI: 3.81–95.57), respectively, compared with the lowest teritle (ptrend < 0.001). When both cohort studies were combined, individuals in the second and third tertile of serum IL-27 had an OR of 6.10 (95% CI: 2.01–18.48) and 26.20 (95% CI: 7.49–91.63) for HCC, respectively, compared with those in the lowest IL-27. The levels of serum IL-6, HMGB1 and sRAGE were not associated with risk of HCC in either the Singapore or the Shanghai cohort study or both cohort studies combined (all ptrend’s >0.40) (Table 3).
Table 3.
Serum IL-27, IL-6, HMGB1 and sRAGE levels in relation to risk of hepatocellular carcinoma, The Singapore Chinese Health Study and The Shanghai Cohort Study separately and combined
| Singapore Cohort Study | Shanghai Cohort Study | Both Cohort Studies combined | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca/Coa | ORb (95% CI) | Ca/Coa | ORb (95% CI) | Ca/Coa | ORb (95% CI) | |||||
| IL-27 | ||||||||||
| 1st tertilec | 15/33 | 1.00 | 11/33 | 1.00 | 26/66 | 1.00 | ||||
| 2nd tertile | 26/34 | 8.74 (1.03–74.06) | 21/34 | 5.42 (1.33–22.07) | 47/68 | 6.10 (2.01–18.48) | ||||
| 3rd tertile | 59/33 | 46.08 (4.68–453.86) | 68/33 | 19.09 (3.81–95.57) | 127/66 | 26.20 (7.49–91.63) | ||||
| P for trend | <0.001 | <0.001 | <0.001 | |||||||
| IL-6d | ||||||||||
| 1st tertile | 29/33 | 1.00 | 29/33 | 1.00 | 58/66 | 1.00 | ||||
| 2nd tertile | 37/34 | 1.04 (0.34–3.22) | 35/32 | 0.95 (0.34–2.66) | 72/66 | 0.98 (0.46–2.09) | ||||
| 3rd tertile | 34/33 | 1.09 (0.32–3.75) | 33/32 | 0.75 (0.25–2.29) | 67/65 | 0.89 (0.39–2.02) | ||||
| P for trend | 0.891 | 0.618 | 0.775 | |||||||
| HMGB1d | ||||||||||
| 1st tertile | 35/33 | 1.00 | 46/33 | 1.00 | 81/66 | 1.00 | ||||
| 2nd tertile | 32/34 | 1.03 (0.44–2.42) | 30/32 | 0.76 (0.31–1.87) | 62/66 | 0.89 (0.48–1.65) | ||||
| 3rd tertile | 33/33 | 0.95 (0.37–2.43) | 21/32 | 0.68 (0.25–1.83) | 54/65 | 0.80 (0.41–1.55) | ||||
| P for trend | 0.916 | 0.433 | 0.506 | |||||||
| sRAGEd | ||||||||||
| 1st tertile | 31/33 | 1.00 | 39/33 | 1.00 | 70/66 | 1.00 | ||||
| 2nd tertile | 43/34 | 1.68 (0.51–5.49) | 34/32 | 1.04 (0.44–2.48) | 77/66 | 1.25 (0.62–2.50) | ||||
| 3rd tertile | 26/33 | 0.94 (0.24–3.72) | 24/32 | 0.70 (0.20–2.46) | 50/65 | 0.76 (0.32–1.82) | ||||
| P for trend | 0.521 | 0.658 | 0.511 | |||||||
Number of cases/number of controls.
Odds ratios (95% confidence intervals) were derived from conditional logistic regression models that also included variables for body mass index (<18.5, 18-<23, 23-<27.5, ≥27.5 kg/m2), alcohol consumption (nondrinkers, moderate drinkers, and heavy drinkers), cigarette smoking (never, former, current smokers), and serological status of hepatitis B surface antigen or antibodies to hepatitis C virus (negative, positive).
See cutoff values of tertile for each biomarker within a given cohort studyin Supplementary Table 1.
Three case-control pairs in the Shanghai Cohort Study were excluded from these analyses due to missing data.
Hepatitis B is the major causal factor for HCC in our study populations. Among the Singapore controls and the Shanghai HCC cases, IL-27 levels were significantly higher in individuals positive for HBsAg than in those negative for HBsAg (Supplementary Tables S2 and 3). We examined the potential synergistic effect of HBsAg and IL-27 on the risk of HCC development in both cohort studies combined. Among HBsAg-negative individuals, the second and third tertile of serum IL-27 were associated with 10.33 (95% CI: 2.21–48.27) and 42.47 (95% CI: 8.30-217-40) times the risk of HCC, respectively, compared with the first tertile (Table 4 and Figure 1). Among individuals with positive HBsAg, the risk of HCC increased with increasing levels of IL-27; those with the highest tertile of IL-27 had an OR of 242.46 (95% CI: 38.42–1529.01) for HCC compared with the lowest tertile of IL-27 and negative HBsAg, although the test for interaction effect between HBsAg and IL-27 on HCC risk was not statistically significant (p for interaction = 0.325).
Table 4.
Joint effect of serum interleukin-27 (IL-27) and chronic infection with hepatitis B virus on risk of hepatocellular carcinoma development in both the Singapore Chinese Health Study and The Shanghai Cohort Study combined
| HBsAg-negative | HBsAg-positive | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | ORa (95% CI) | Cases | Controls | ORa (95% CI) | ||
| IL-27 level | |||||||
| 1st tertile | 15 | 63 | 1.00 (referent) | 11 | 3 | 23.91 (3.17–180.47) | |
| 2nd tertile | 29 | 62 | 10.33 (2.21–48.27) | 18 | 6 | 61.33 (9.66–389.57) | |
| 3rd tertile | 62 | 55 | 42.47 (8.30–217.40) | 65 | 11 | 242.46 (38.42–1529.01) | |
Odds ratios (95% confidence intervals) were calculated for each of joint exposure levels relative to the lowest tertile of IL-27 and hepatitis B surface antigen (HBsAg) negative group using conditional logistic regression model that also included variables for body mass index (<18.5, 18-<23, 23-<27.5, ≥27.5 kg/m2), alcohol consumption (nondrinkers, moderate drinkers, and heavy drinkers), and cigarette smoking (never, former, current smokers).
Figure 1. Odds ratio of hepatocellular carcinoma (HCC) by serum levels of interlekin-27 (IL-27) and serological status of hepatitis B surface antigen (HBsAg) in the Singapore and Shanghai cohort studies.
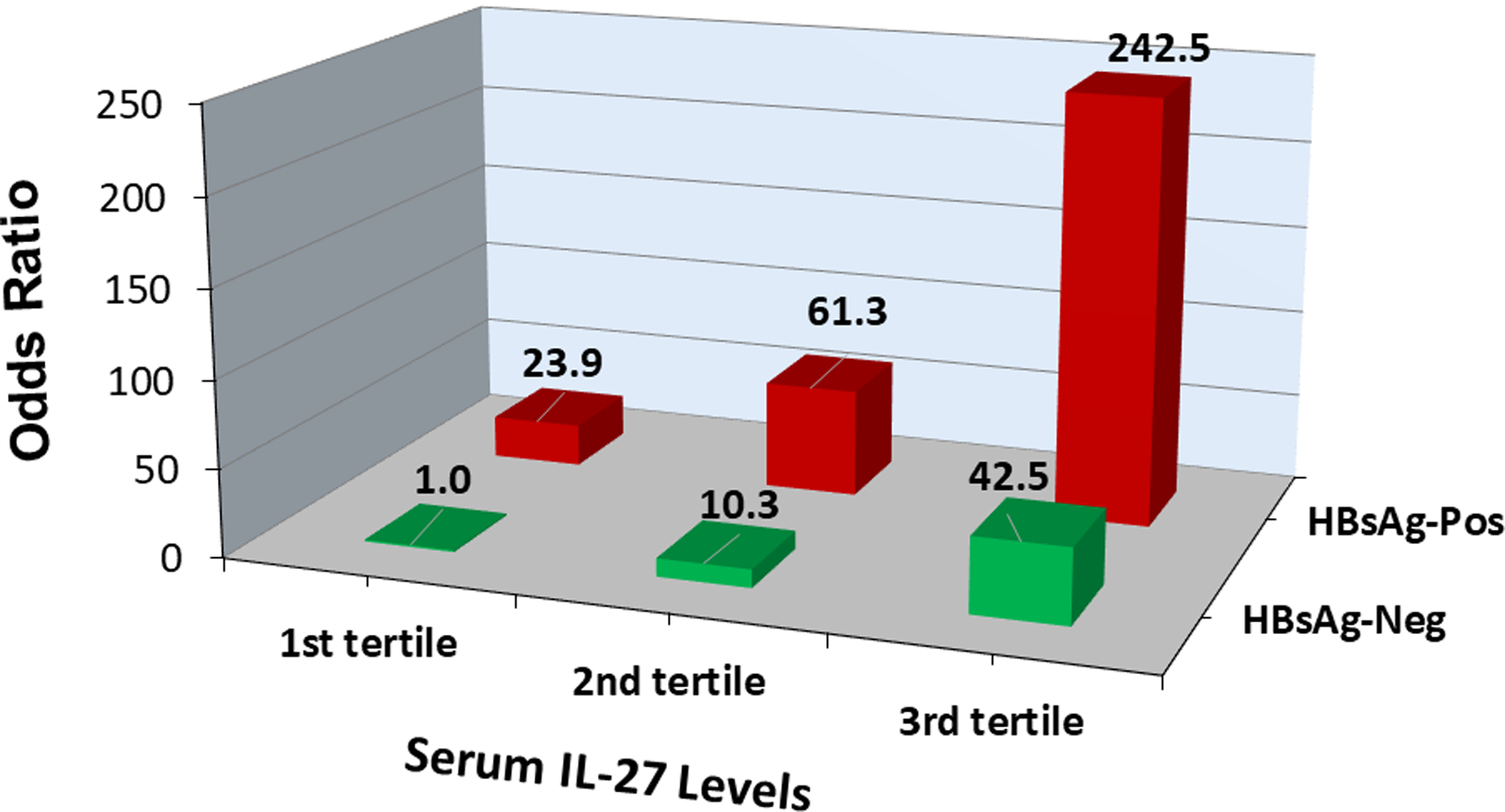
Odds ratio for HCC increased with increasing IL-27 levels among HBsAg-negative or HBsAg-positive individuals.
IL-27 concentrations were higher in women than men in either HCC cases (3.08 versus 2.16 nl/mL, p = 0.006) or controls (2.07 versus 1.69 ng/mL, p = 0.136) of the Singapore cohort study, although the difference reached statistical significance within HCC cases only. In the stratified analysis, IL-27 levels were statistically significantly higher in HCC cases than controls in both men and women separately (both p’s = 0.008) (Supplementary Table S2).
The association between serum levels of IL-27 and the time interval from blood collection to the diagnosis of HCC is shown in Table 5. The IL-27 level increased with the decreasing time interval in both the Singapore and the Shanghai cohort studies, and the trend was statistically significant in both cohort studies combined (ptrend = 0.001).
Table 5.
Serum concentration of interleukin-27 (IL-27) by the time interval from blood collection to diagnosis of hepatocellular carcinoma, The Singapore Chinese Health Study and The Shanghai Cohort Study separately and combined
| Time interval (yr) | Singapore Cohort Study | Shanghai Cohort Study | Both Cohort Studies combined | |||||
|---|---|---|---|---|---|---|---|---|
| N | IL-27 (ng/ml)a | N | IL-27a | N | IL-27 (ng/ml)a | |||
| 0.3–<3 | 29 | 2.86 (2.36–3.46) | 37 | 3.32 (2.66–4.12) | 66 | 3.22 (2.78–3.72) | ||
| 3–<6 | 41 | 2.12 (1.80–2.50) | 26 | 2.60 (2.02–3.36) | 67 | 2.40 (2.08–2.76) | ||
| 6–10 | 30 | 2.20 (1.82–2.66) | 37 | 2.44 (1.96–3.02) | 67 | 2.26 (1.94–2.62) | ||
| P trend | 0.072 | 0.062 | 0.001 | |||||
Geometric means were derived from analysis of covariance with following covariates: study location (Shanghai vs Singapore), sex, body mass index (<18.5, 18-<23, 23-<27.5, ≥27.5 kg/m2), alcohol consumption (nondrinkers, moderate drinkers, and heavy drinkers), and cigarette smoking (never, former, current smokers), and serological status of hepatitis B surface antigen or antibodies to hepatitis C virus (negative, positive).
Serum IL-27 levels were not correlated with serum IL-6, HMGB1, and sRAGE (all rho’s < 0.1, p’s > 0.18) among all control subjects. Among controls of the Singapore cohort study, BMI was inversely associated with serum IL-27 (Supplementary Table S2). Among controls of the Shanghai cohort study, high alcohol consumption was related to lower serum IL-27 (Supplementary Table S3). We did not find any statistically significant association for any other variables studied with IL-27 (Supplementary Tables S2 and S3).
DISCUSSION
The present studies demonstrate that IL-27 in baseline sera predicts the risk of developing HCC in the next 5 years. There was a 46-fold increased risk of HCC development for individuals with the highest tertile of IL-27 compared with the lowest IL-27 in the Singapore Chinese Health Study. These initial findings in the Singapore Chinese Health Study were validated in the Shanghai Cohort Study. The strong dose-dependent positive association between serum IL-27 and HCC risk was independent of chronic infection with HBV or HCV, alcohol consumption, cigarette smoking and obesity.
Chronic infection with HBV is the strongest risk factor for HCC in our study populations whereas HCV infection is rare. In vitro experiments have demonstrated that HBV infection activated IL-27 gene expression in hepatocytes or human monocytic cells (27, 28). The mRNA expression levels of IL-27 gene were significantly elevated in patients infected with HBV than those without HBV infection (28, 29). In addition, IL-27 gene is overexpressed in tumor tissue of HCC than in tumor tissues of any other cancer types in The Cancer Genome Atlas (TCGA). These data suggest that the liver is a probable source of the elevated serum IL-27 in HCC patients.
Several hospital-based case-control studies have shown that serum IL-27 levels were higher in patients with viral hepatitis than healthy controls, higher in patients with cirrhosis or HCC than those with viral hepatitis without cirrhosis or HCC, and higher in patients with advanced stage of HCC than in those with early stage of HCC (28, 30, 31). Consistent with these previous studies, our studies demonstrate that IL-27 levels are elevated in sera collected from individuals, on average, 4–5 years prior to the diagnosis of HCC.
In our study populations, approximately 50% of HCC cases tested negative for both HBsAg and anti-HCV. For these non-viral related HCC, we previously found that dietary exposure to aflatoxin B1 contributed to the risk of developing HCC in the Shanghai Cohort Study (20, 32). Although overall BMI level was lower in our study populations than Americans, the prevalence of obesity and type 2 diabetes, the underlying causes of NAFLD, in participants of the Singapore Chinese Health Cohort study were comparable with the corresponding figures in the US in the early 1990s (33), suggesting that NAFLD may play a more prominent role in the development of non-viral related HCC in Singapore. Type 2 diabetes has been found to be a risk factor for HCC in the Singapore cohort study (34). A stronger association for serum IL-27 with risk of non-viral related HCC than viral-related HCC suggest that IL-27 may play an important and systemic role in the development of HCC regardless of its underlying causes.
It is unique for IL-27 that has both pro- and anti-inflammatory properties. Early studies have shown that IL-27 can promote Th1 responses by activating naïve CD4+ T cells and natural killer cells to produce interferon-γ. This process drives inflammation. Contrarily, IL-27 can suppress autoimmune and inflammatory conditions by stimulating CD4+ T cells to express immune-regulatory cytokine IL-10 (35). IL-27 also promotes expression of inhibitory receptors including PD-1, PD-L1, LAG3, TIGIT and TIM3 on T cells, that subsequently limit immune responses (18, 19). The liver-resident plasmacytoid dendritic cells had significantly higher gene expression of IL-27 than the splenic plasmacytoid dendritic cells (36). The profound elevation of IL-27 mRNA in the liver tumor tissues than in any other cancer type in TCGA further supports the notion that IL-27 is highly enriched in the liver and may play an important role in the immunosuppression in the tumor microenvironment that promotes the development of HCC.
Our finding that there is no evidence of elevated serum HMGB1, sRAGE, or IL-6 in HCC are also important, since it suggests that these proximal biomarkers are below the level of detection, perhaps in part due to more rapid clearance. Furthermore, it suggests that like IL-12 (37–39), IL-27 has a prolonged half-life allowing its persistence not only at sites where it is generated, such as the liver, but also within the circulation, allowing for its utility as a sensitive biomarker of chronic inflammation and risk of subsequent development of HCC.
The strengths of the study include the prospective study design in which serum samples were collected, on average, 4.5 years prior to the diagnosis of HCC. Thus, the measured biomarkers were not impacted by the progress of liver disease or the status of HCC, which is inherent in a cross-sectional case-control study where blood samples are collected at or after HCC diagnosis. The validation of the initial findings by an independent cohort study strongly implicates an important role of IL-27 in the development of HCC. Although IL-27 was measured in serum, the significantly elevated levels of IL-27 mRNA in the tumor tissue of HCC relative to tumor tissues of other cancer types in the TCGA project indicates that the liver is probably the main source of the elevated serum IL-27 in HCC cases. The chief limitation of the study was relatively small sample size, especially number of healthy control subjects with positive HBsAg, which limited the statistical power to detect a statistically significant synergistic effect for IL-27 with HBV infection on HCC risk. Another limitation is that we did not measure the fibrosis or cirrhosis status of the cohort study participants at enrollment, which was impractical and too costly given such large sample sizes of the two cohort studies. We also analyzed serum IL-27 in 60 patients with biopsy-proven non-alcoholic steatohepatitis (NASH) in Pittsburgh, Pennsylvania. Of the 60 NASH patients, 17 had no or mild fibrosis, 20 had severe fibrosis, and 23 had advanced fibrosis or cirrhosis. A moderate positive relationship between serum IL-27 and fibrosis stage (ρ = 0.256, p = 0.049) was observed. These results support the notion that IL-27 elevation occurred long before the development of HCC since liver fibrosis takes place many years prior to HCC development and is a strong risk determinant for HCC (40, 41).
In summary, the present study discovered and validated the observation that serum IL-27 levels were significantly elevated in persons, on average, 4–5 years prior to their diagnosis of HCC. The main source of elevated serum IL-27 is the liver. A strong positive association between serum IL-27 and the risk of developing HCC regardless of underlying risk factors suggest that IL-27 has a broad role, possibly through the immunosuppression and tumor immune escape mechanism, in the development of HCC. Given that IL-27 can promote the expression of inhibitory receptors on immune cells, our findings also suggest that anti-IL-27 may be a potential strategy for immunoprevention against the development of HCC in high-risk individuals.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Singapore Cancer Registry and the Shanghai Cancer Registry for the identification of incident cancer cases among participants of the Singapore Chinese Health Study and the Shanghai Cohort Study, respectively, via database linkage. We also thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study, and Xue-Li Wang of the Shanghai Cancer Institute for supervising the field work of the Shanghai Cohort Study.
Grant support: Research reported in this publication was supported by the National Cancer Institute (NCI) of the National Institutes of Health under award numbers: R01CA144034 and UM1CA182876 (both to J-M Yuan). Serum biomarkers were measured in the Biomarker Shared Resource of the UPMC Hillman Cancer Center, supported in part by NCI under award number: P30CA067904 (to Robert L. Ferris).
Abbreviations:
- CI
confidence interval
- DAMP
damage associated molecular pattern
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HMGB1
high mobility group box 1
- IL
interleukin
- OR
Odds ratio
- PAMP
pathogen associated molecular pattern
- RAGE
receptor for advanced glycation endproducts
- TCGA
The Cancer Genome Atlas
Footnotes
Conflicts of interest: The authors declare no potential conflicts of interest.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–76. [DOI] [PubMed] [Google Scholar]
- 3.Yu MC, Yuan J-M, Govindarajan S, Ross RK. Epidemiology of hepatocellular carcinoma. Can J Gastroenterol 2000;14:703–9. [DOI] [PubMed] [Google Scholar]
- 4.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 2019;16:411–28. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global hepatitis report 2017. Geneva: World Health Orgnization, 2017; p. 7 [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 7.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012;10:1342–59 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol 2016;13:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuai Z, Leung MW, He X, Zhang W, Yang G, Leung PS, et al. Adaptive immunity in the liver. Cell Mol Immunol 2016;13:354–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabbi M, Carbotti G, Ferrini S. Dual Roles of IL-27 in Cancer Biology and Immunotherapy. Mediators Inflamm 2017;2017:3958069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002;16:779–90. [DOI] [PubMed] [Google Scholar]
- 12.Kamakura M, Morisawa K, Komi H, Tomatani A, Saito F, Konishi Y, et al. Regulation of IL-27p28 gene by lipopolysaccharide in dendritic DC2.4 cells. Biochem Biophys Res Commun 2006;349:1372–7. [DOI] [PubMed] [Google Scholar]
- 13.Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, et al. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol 2007;178:7607–15. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth E, Baird AW, O’Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol 2009;31:333–43. [DOI] [PubMed] [Google Scholar]
- 15.Horst AK, Neumann K, Diehl L, Tiegs G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol 2016;13:277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 2012;37:511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol 2013;190:1510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018;558:454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLong JH, O’Hara Hall A, Rausch M, Moodley D, Perry J, Park J, et al. IL-27 and TCR Stimulation Promote T Cell Expression of Multiple Inhibitory Receptors. Immunohorizons 2019;3:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross RK, Yuan J-M, Yu MC, Wogan GN, Qian GS, Tu JT, et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 1992;339:943–6. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J-M, Stram DO, Arakawa K, Lee HP, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev 2003;12:890–8. [PubMed] [Google Scholar]
- 22.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 2001;39:187–95. [DOI] [PubMed] [Google Scholar]
- 23.Yuan J-M, Ross RK, Wang XL, Gao YT, Henderson BE, Yu MC. Morbidity and mortality in relation to cigarette smoking in Shanghai, China. A prospective male cohort study. JAMA 1996;275:1646–50. [PubMed] [Google Scholar]
- 24.Yuan J-M, Ross RK, Stanczyk FZ, Govindarajan S, Gao YT, Henderson BE, et al. A cohort study of serum testosterone and hepatocellular carcinoma in Shanghai, China. Int J Cancer 1995;63:491–3. [DOI] [PubMed] [Google Scholar]
- 25.Koh WP, Robien K, Wang R, Govindarajan S, Yuan J-M, Yu MC. Smoking as an independent risk factor for hepatocellular carcinoma: the Singapore Chinese Health Study. Br J Cancer 2011;105:1430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Expert Consultation WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Zhang R, Zhang W, Zhu C, Yu Y, Song Y, et al. IL-27, a cytokine, and IFN-lambda1, a type III IFN, are coordinated to regulate virus replication through type I IFN. J Immunol 2014;192:691–703. [DOI] [PubMed] [Google Scholar]
- 28.Zhu C, Zhang R, Liu L, Rasool ST, Mu Y, Sun W, et al. Hepatitis B virus enhances interleukin-27 expression both in vivo and in vitro. Clin Immunol 2009;131:92–7. [DOI] [PubMed] [Google Scholar]
- 29.Zare A, Karimi MH, Rashki A, Geramizadeh B, Afshari A, Miri HR, et al. Association of the Interleukin-27 Gene Expression and Hepatitis B Virus Infection in Liver Transplanted Patients. Exp Clin Transplant 2017;15:554–60. [DOI] [PubMed] [Google Scholar]
- 30.Kao JT, Lai HC, Tsai SM, Lin PC, Chuang PH, Yu CJ, et al. Rather than interleukin-27, interleukin-6 expresses positive correlation with liver severity in naive hepatitis B infection patients. Liver Int 2012;32:928–36. [DOI] [PubMed] [Google Scholar]
- 31.Zhang GL, Xie DY, Ye YN, Lin CS, Zhang XH, Zheng YB, et al. High level of IL-27 positively correlated with Th17 cells may indicate liver injury in patients infected with HBV. Liver Int 2014;34:266–73. [DOI] [PubMed] [Google Scholar]
- 32.Qian GS, Ross RK, Yu MC, Yuan J-M, Gao YT, Henderson BE, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol Biomarkers Prev 1994;3:3–10. [PubMed] [Google Scholar]
- 33.Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564–8. [DOI] [PubMed] [Google Scholar]
- 34.Koh WP, Wang R, Jin A, Yu MC, Yuan J-M. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer 2013;108:1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol 2015;33:417–43. [DOI] [PubMed] [Google Scholar]
- 36.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol 2012;188:5227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lotze MT, Finn OJ. Report on the Keystone symposium: cellular immunity and the immunotherapy of cancer II. J Immunother Emphasis Tumor Immunol 1993;14:79–87. [PubMed] [Google Scholar]
- 38.Lotze MT, Hellerstedt B, Stolinski L, Tueting T, Wilson C, Kinzler D, et al. The role of interleukin-2, interleukin-12, and dendritic cells in cancer therapy. Cancer J Sci Am 1997;3 Suppl 1:S109–14. [PubMed] [Google Scholar]
- 39.Popovic PJ, DeMarco R, Lotze MT, Winikoff SE, Bartlett DL, Krieg AM, et al. High mobility group B1 protein suppresses the human plasmacytoid dendritic cell response to TLR9 agonists. J Immunol 2006;177:8701–7. [DOI] [PubMed] [Google Scholar]
- 40.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology 2011;53:1874–82. [DOI] [PubMed] [Google Scholar]
- 41.O’Rourke JM, Sagar VM, Shah T, Shetty S. Carcinogenesis on the background of liver fibrosis: Implications for the management of hepatocellular cancer. World J Gastroenterol 2018;24:4436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


