Figure 5. Mucolytic therapy decreases mucinous tumor growth in a rat PDX model of late (advanced) MCP.
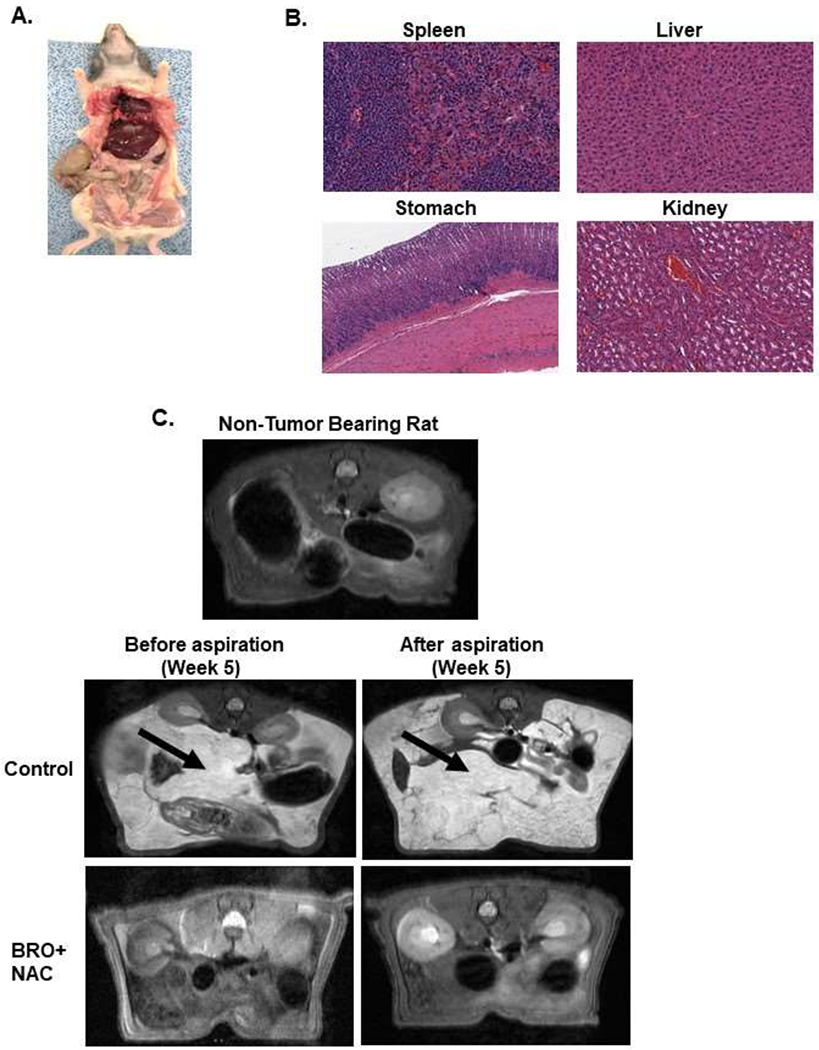
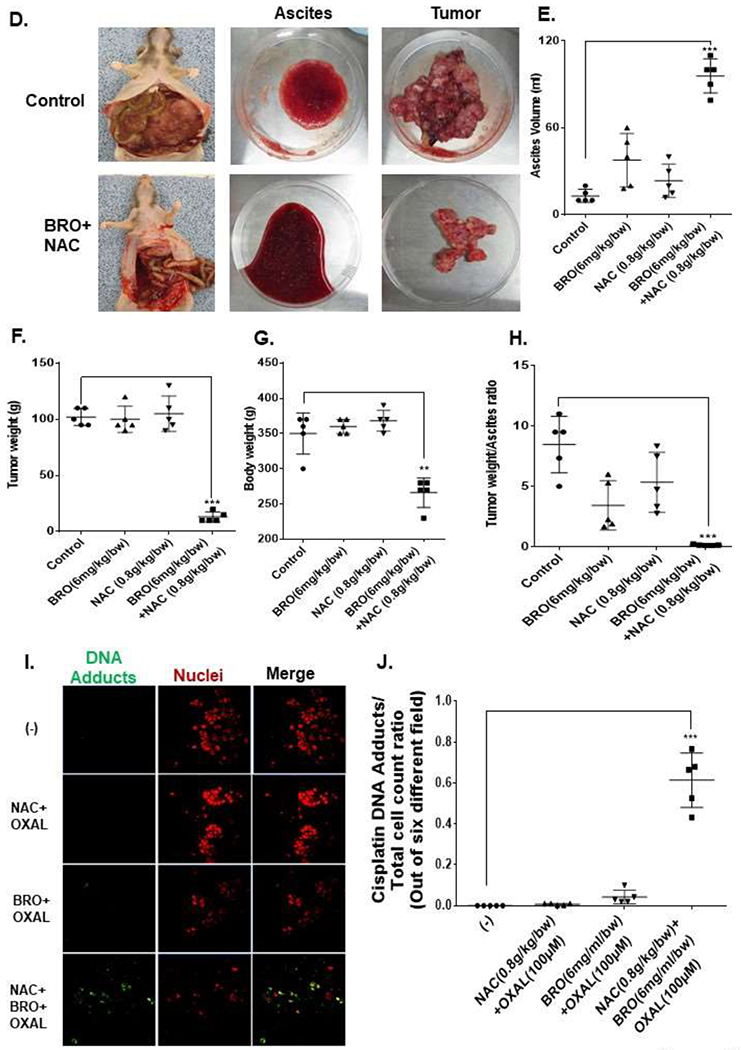
Non-tumor bearing nude rats were administered 5 ml of BRO (6 mg/kg bw) + NAC (0.8 g/kg bw) solution (in PBS, pH 8.0) or PBS (control) IP every other day for 2 weeks; gross evaluation (A) and pathological microscopic evaluation of abdominal organs (with H&E staining) (B) for toxicity is shown. Late (advanced) MCP model of IP mucolytic (versus control) therapy at same dose/frequency as in toxicity studies; pre-and post ascites drainage MRIs at week 5 (MRI of non-tumor bearing rat is also shown) (C); gross pictures of mucinous ascites drained and residual abdominal mucinous tumor at sacrifice (week 5) (D); volume of muinous ascites (E), residual mucinous tumor weight (F), gross body weight (G), and ratio of ascites volume to tumor weight (H). Residual mucinous tumor fragments following IP mucolytic (or control) therapy, treated with OXAL (100 μM) for 24h ex vivo, and subjected to IF assay to quantify DNA adducts (I, J). Error bars represent standard deviation (SD) from triplicate experiments (**P< .01, ***P< .001).
