Table 3.
Synthesis of isochromans and tethrahydroisoquinolines by enantioselective C–H insertion
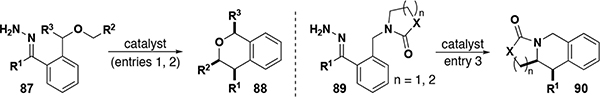 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | X | catalyst | # examples | yield (%) | dr (cis.trans) | er | ref. |
| 1 | Ar,[a] CH3 | Ar,[b] alkyl, alkenyl, propargyl | H | NA | [Rh], 17 | 18 | 54–98 | >95:5 | 63:37 to >99.5:0.5 | 60 |
| 2 | Ph | PMP, alkenyl | Ph, CH3 | NA | [Rh], 22, 15 | 3 | 60–70 | >95:<1 :<1 :<1 | NA | 60 |
| 3 | Ph | NA | H | O, CH2 | [Rh], 17 | 3 | 54–83 | >95:5 | 94:6 to 99:1 | 60 |
Ar = Ph, substituted Ph, 3-pyridyl.
Ar = Ph, substituted Ph
