Table 4.
N–H insertion reaction scope with donor/donor metal carbenes
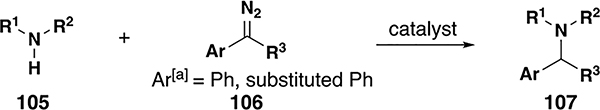 | |||||||
|---|---|---|---|---|---|---|---|
| entry[b] | R1 | R2 | R3 | catalyst | # examples | yield (%) | ref. |
| 1 | aryl | H, alkyl | H, alkyl, aryl | [Cu], 32 | 40 | 50–93 | 18 |
| 2 | alkyl | H, alkyl | aryl | [Ru], 29 | 10 | 10–48 | 65 |
| 3 | alkyl | H, alkyl | H, alkyl, aryl | [Cu], 30, 32 | 23 | 40–84 | 18,41,67 |
| 4[c] | imidazoles | H, aryl | [Cu], 32 | 10 | 50–80 | 66 | |
| 5[d] | COR | H | H | [Cu], 33 | 38 | 33–99 | 68 |
| 6 | COR | H, alkyl, aryl | alkyl/aryl | [Cu], 31, 33 | 38 | 40–98 | 42,68 |
| 7 | imidazole, carbazole, morpholine | Ph | [Fe], 38 | 3 | 86–94 | 64 | |
| 8 | Ph, Bn | H | Ph | [Fe], 38 | 2 | 51–56 | 64 |
includes Ar = 2-pyridyl napthyl (entry 1); Ar/R3 = fluorenyl (entry 2); Ar = napthyl (entries 5 and 6).
diazo compounds for all entries except 7 and 8 are derived from tosylhydrazones
includes 2 examples with cyclohexanone-derived tosylhydrazones.
includes 3 examples with tosylhydrazones derived from alkyl aldehydes.
