Table 5.
O–H and S–H insertion reaction scope
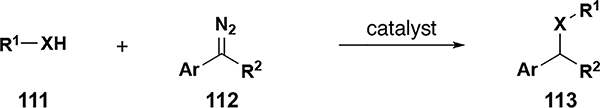 | ||||||
|---|---|---|---|---|---|---|
| entry[a] | X | R1 | Ar, R2 | # examples | yield (%) | ref. |
| 1[b] | O | CH3 | Ar, R2 = fluorenyl | 1 | 100 | 69 |
| 3[e] | O | Ph, Ar, 2-napthyl, alkyl | Ar, R2 = Ph | 5 | 31–92 | 64 |
| 4[e] | S | Ph, Ar, 2-pyridyl, alkyl | Ar, R2 = Ph | 5 | 33–99 | 64 |
diazo compounds derived from tosylhydrazones for entries 1 and 2.
cis-[Pt(PPh3)2(CH3CN)2][BF4]2 41 catalyst
Fe(TPFPP)Ad 38 as catalyst
