Table 7.
Stereoselective Si–H insertion reactions with donor/donor carbenes
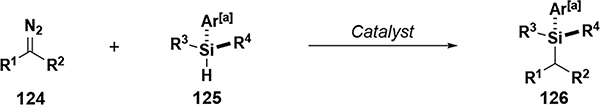 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | R4 | dr | er | catalyst | # examples | yield (%) | Ref. |
| 1 | Ar | R1 | alkyl, siloxy | H | NA | 50:50 to 86:14[b] | [Rh], 15 | 15 | 45–91 | 74 |
| 2 | Ar | Ar ≠ R1 | H, alkyl, aryl | H | 61:39 to 95:5 | 61:3 to 95:5[b,c] | [Rh], 15 | 9 | 55–95 | 74 |
| 3 | Ar | Ar ≠ R1 | CH3, Et, siloxy | R3 | NA | 68:32 to 99.5:0.5[c] | [Rh], 25 | 49 | 36–96 | 75 |
Ar = Ph, substituted Ph, napthyl, indolyl.
er denotes chirality at silicon.
er denotes chirality at carbon.
