Table 9.
Diazo-free C–H insertion reactions with donor/donor and donor carbenes from enynones
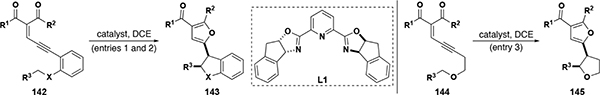 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| entry | X | R1 | R2 | R3 | catalyst | # examples | yield (%) | dr | er | ref |
| 1[a] | O, NAc | alkyl, alkoxy | alkyl | alkyl, alkenyl, CO2Et, Ar[b] | [Rh], 17 | 29 | 65–99 | 50:50 to >99:1 | 82:18 to 99.5:0.5 | 79 |
| 2[c] | NAc | alkyl,alkoxy, Ph | alkyl, Ph | alkyl, alkenyl, Ar[b] | [Ru], 26 | 34 | 43–97 | 91:9 to >99:1 | 98:2 to >99.5:0.5 | 80 |
| 3[a] | NA | CH3 | CH3 | alkenyl, Ph | [Rh], 17 | 3 | 99 | 77:23 to 95:5 | 80:20 to 87:13 | 79 |
reactions run −20 to −30 °C.
Ar = Ph, substituted Ph, napthyl (entry 1); Ar = Ph, substituted Ph (entry 2).
reactions run 80–120 °C
