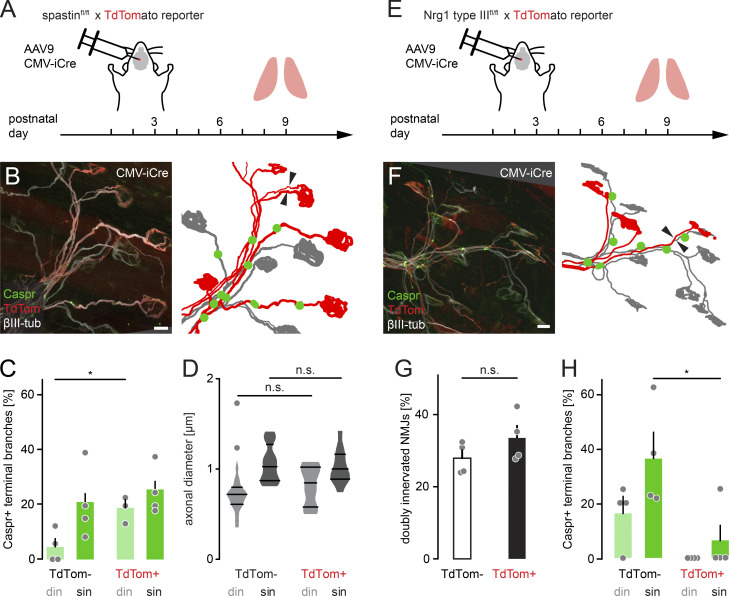
Figure S3.
AAV9-mediated spastin deletion promotes myelination on competing branches. (A) Schematic of experimental design. AAV9-CMV-iCre was injected at P2 into the third ventricle of spastinfl/fl × TdTomato reporter mice. Muscles were analyzed at P9. (B) Image of P9 muscle immunostained for Caspr (green) and βIII-tubulin (white). iCre-mediated deletion resulted in TdTomato-positive axons (red), presumed to lack spastin. Schematic on the right depicts TdTomato-positive (red) and negative motor units (gray) and Caspr paranodes (green). Arrowheads point to competing axons leading to the same NMJ. (C) Quantification of Caspr immunostaining on TdTomato-negative and positive terminal branches at P9 (n ≥ 3 mice per group, n ≥ 15 axons per mouse). (D) Quantification of axon diameter of TdTomato-negative and positive terminal branches at P9 (n ≥ 10 axons per group, n = 5 mice). (E) Schematic of experimental design. AAV9-CMV-iCre was injected at P2 into the third ventricle of Nrg1 type IIIfl/fl × TdTomato reporter mice. Muscles were analyzed at P9. (F) Image of P9 muscle immunostained for Caspr (green) and βIII-tubulin (white). iCre-mediated deletion resulted in TdTomato-positive axons (red), presumed to lack Nrg1. Schematic on the right depicts TdTomato-positive (red) and negative motor units (gray) and Caspr paranodes (green). Arrowheads point to two axons leading to the same NMJ. (G) Quantification of doubly innervated NMJs on TdTomato-negative and positive terminal branches at P9 (n = 4 mice per group, ≥97 axons per animal). (H) Quantification of Caspr immunostaining on TdTomato-negative and positive terminal branches at P9 (n = 4 mice per group, ≥29 axons per animal). din, competing axons; sin, winner axons. Data represent mean + SEM. *, P < 0.05; n.s., not significant; Mann–Whitney test. Outlier determined by Tukey test. Scale bar, 10 µm (B and F).