Figure 1.
Celastrol protects C57BL/6 mice from HFD-induced obesity
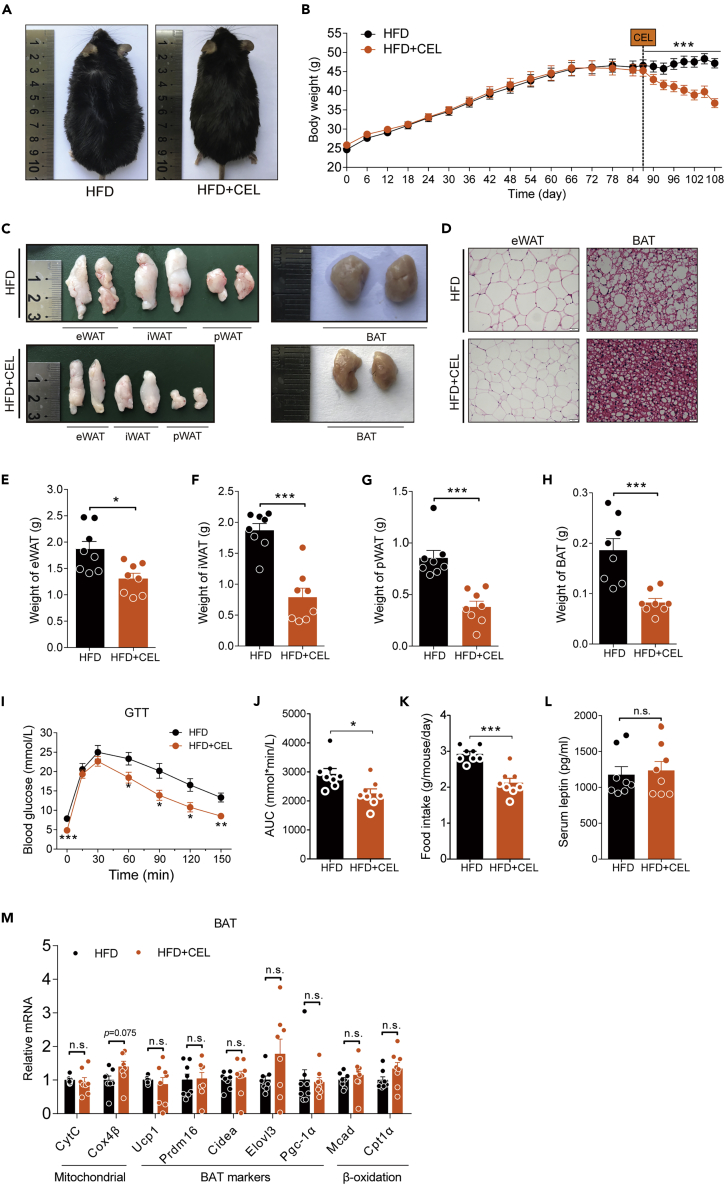
(A–M) HFD-fed obese C57BL/6 mice were subjected to oral administration of celastrol (3 mg/kg/day) for 3 weeks. (A) Representative pictures of male DIO C57BL/6 mice after 3 weeks of celastrol treatment. (B) BW of DIO mice during the treatment (n = 8 for each group). (C) Macroscopic view of representative sections of epididymal fat depots (eWAT), inguinal fat depots (iWAT), perirenal fat depots (pWAT), and brown adipose tissue (BAT) of mice treated with celastrol or not. (D) Representative H&E-stained images of eWAT (200×) (Scale bar, 50 μm) and BAT (400×) (Scale bar, 20 μm). (E–H) Weights of (E) eWAT, (F) iWAT, (G) pWAT, and (H) BAT of mice treated with celastrol or not (n = 8 for each group). (I–J) Results for (I) intraperitoneal glucose tolerance tests (GTT) and (J) the area under the curve (AUC) for GTTs (n = 8 for each group). (K) Three-day average food intake of DIO mice during celastrol treatment (n = 8 for each group). (L) Serum leptin levels of mice treated with celastrol or not (n = 8 for each group). (M) Gene expression analysis of mitochondrial and brown fat gene programs in BAT of mice treated with celastrol or not (n = 8 for each group). Error bars represent the mean ± SEM. p values were determined by two-way ANOVA (B) or Student's t test. ∗p < 0.05, ∗∗∗p < 0.001; n.s., not significant (p > 0.05).