Abstract
Objective
We aimed to clarify the cognitive function of patients with chronic obstructive pulmonary disease (COPD) and different nutritional status.
Methods
Among 95 patients with COPD in this retrospective study, we administered the Nutritional Risk Screening 2002 (NRS 2002) and Mini-Mental State Examination (MMSE). We recorded patients’ clinical characteristics, comorbidities, and laboratory measurements. According to NRS 2002 scores, patients were divided into two groups: no nutritional risk with NRS 2002 < 3 (n = 54) and nutritional risk, with NRS 2002 ≥ 3 (n = 41).
Results
We found a negative correlation between NRS 2002 and MMSE scores in participants with COPD (r = −0.313). Patients with nutritional risk were more likely to be cognitively impaired than those with no nutritional risk. Multivariate logistic regression analysis indicated that malnutrition was an independent risk factor for cognitive impairment, after adjusting for confounders (odds ratio [OR] = 4.120, 95% confidence interval [CI]: 1.072–15.837). We found a similar association between NRS 2002 and MMSE scores at 90-day follow-up using a Pearson’s correlation test (r = −0.493) and logistic regression analysis (OR = 7.333, 95% CI: 1.114–48.264).
Conclusions
Patients with COPD at nutritional risk are more likely to have cognitive impairment.
Keywords: Nutritional risk, cognitive function, chronic obstructive pulmonary disease, Mini-Mental State Examination, Nutritional Risk Screening 2002, lung disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and restricted airflow, usually associated with considerable exposure to harmful particles or gases, resulting in airway and/or alveolar abnormalities. As the disease progresses, ventilatory dysfunction causes the retention of oxygen and carbon dioxide, varying degrees of hypoxemia and hypercapnia, and eventually respiratory failure. It is now widely recognized that COPD comorbidities aggravate the progression of disease and reduce patients’ quality of life1,2 and can lead to the development of cognitive impairment.3,4 Hypoxemia, serum clusterin levels, depression, and anxiety are associated with cognitive impairment in patients with COPD.5,6 However, the mechanism of cognitive decline and its related factors have not been rigorously studied.7
Recent studies have reported that malnutrition plays an important role in cognitive impairment.8 Several studies have shown that there is a relationship between nutrition and cognition in some diseases, such as Alzheimer disease and Parkinson disease.9,10 However, data remain scarce regarding whether malnutrition contributes to the decline in cognition among patients with COPD. In this paper, we examined the association between nutritional risk and cognitive function in patients with COPD.
Methods
Participants
Between February 2018 and February 2019, we performed a retrospective study among patients with COPD from the respiratory ward of the Third Affiliated Hospital of Wenzhou Medical University. The inclusion criteria were patients with a diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria and who consented to voluntarily participate in the study.11 Exclusion criteria were: 1) neurological and psychotic disorders, such as psychosis or epilepsy,12 2) cerebral tumor, trauma, or craniotomy,13 3) cerebral hemorrhage, cerebral infarction, or other brain diseases, 4) cancer or pulmonary fibrosis,12 5) and Mini-Mental State Examination (MMSE) and Nutritional Risk Screening 2002 (NRS 2002) evaluations could not be completed or were completed with outliers.
This study was approved by the Institutional Ethics Committee review board of the Third Affiliated Hospital of Wenzhou Medical University and was conducted in accordance with the principles of the Declaration of Helsinki. The Ethics Committee approval number was YJ20170015, and the date of approval was November 24, 2017. All patient details were deidentified. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for observational studies.14
Data collection
Information on age, sex, body mass index (BMI), education level, smoking and drinking habits, duration of disease, and comorbidities were recorded at hospital admission. Blood samples were collected and used to analyze blood routine parameters, blood biochemistry, and arterial blood gas. Laboratory findings at hospital admission were recorded: force expiratory volume in 1 second (FEV1), FEV1 related to the predicted value (FEV1%), and GOLD stage. After providing their informed consent, patients completed all measures, including the BMI, airflow obstruction, dyspnea and exercise capacity (BODE) index; a COPD assessment test (CAT), the Hospital Anxiety and Depression Scale (HAD), Pittsburgh Sleep Quality Index (PSQI), Morse Fall Scale (MFS), MMSE, and NRS 2002. Patients were evaluated upon hospital admission by the study investigators, who also followed up with participants after 3 months.
Diagnostic criteria
Patients were categorized into severity grades I to IV using spirometry (GOLD I: FEV1 ≥80% predicted; GOLD II: 50% ≤FEV1 < 80% predicted; GOLD III: 30% ≤FEV1 <50% predicted; GOLD IV: FEV1 < 30% predicted).15
We measured the cognitive function of patients with COPD using the MMSE, which is one of the most commonly used standardized tools for assessing cognitive function. The total score of this test is 30 points. Scores of 0 to 26 points indicate cognitive impairment, and scores of 27 to 30 points indicate normal cognitive function.16,17
The nutritional status of patients with COPD was evaluated using the NRS 2002. The main purpose of the NRS 2002 is to identify patients who may benefit from nutritional intervention. The NRS 2002 includes three parts: disease status, nutritional status, and age. Scores of 0 to 2 points indicate no nutritional risk whereas scores of 3 to 7 indicate that a nutritional risk exists.18,19
Statistical analyses
All statistical analyses were performed using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Differences between the two groups were analyzed using the chi-square test for categorical variables and the independent samples t-test for continuous variables. Subgroup analysis was performed using the chi-square test. The relationship between MMSE and NRS scores was evaluated with the Pearson’s correlation test. To identify those variables associated with cognitive impairment, data were initially evaluated using univariate logistic regression analysis. To explain the contribution of nutritional risk to cognitive impairment, multivariable models were constructed for multivariate logistic regression analysis, controlling for confounders including age, sex, duration of disease, education, smoking history, albumin, and MFS scores. Statistical significance was set to P < 0.05.
Results
Baseline characteristics of study participants
Among 95 patients with COPD (80 male patients), mean age was 69.94 (SD 8.34) years; 42 (44.21%) were cognitively impaired and 53 (55.79%) were cognitively intact, according to MMSE scores. As for nutritional status assessed using the NRS 2002, 41 participants (43.16%) were at risk of malnutrition and 54 (56.84%) were well nourished.
Based on NRS 2002 scores, the 95 participants were divided into two groups, the group with a risk of malnutrition (NRS 2002 score ≥3) and the well-nourished group (NRS 2002 score < 3). Between the two groups, there were no statistical differences in terms of age, sex, education, smoking, drinking, duration of disease, white blood cells, red blood cells (RBC), hemoglobin, platelets, high-density lipoprotein, and results of the CAT, partial pressure of oxygen in arterial blood (PO2), HAD(A), HAD(D), PSQI, and MFS. However, the values of BMI, albumin, triglycerides, total cholesterol, and low-density lipoprotein, FEV1, and FEV1% of patients with a risk of malnutrition were significantly lower than those of the group with no nutritional risk (P < 0.001, P < 0.001, P = 0.001, P = 0.025, P = 0.010, P < 0.001, and P < 0.001, respectively). In contrast, BODE indexes were significantly higher in patients with a nutritional risk (P < 0.001). Moreover, there were significantly more patients with GOLD stages I or II and normal cognitive function (P = 0.015, P = 0.042, respectively) in the well-nourished group (Table 1).
Table 1.
Characteristics of patients with COPD, according to nutritional status.
| Variable | Well nourished (N = 54) | Risk of malnutrition (N = 41) | P value |
|---|---|---|---|
| Age (years) | 70.83 ± 8.82 | 68.76 ± 7.59 | 0.231 |
| Sex (male), n (%) | 46 (85.19%) | 34 (82.93%) | 0.765 |
| BMI (kg/m2) | 23.65 ± 2.40 | 17.86 ± 1.36 | <0.001 |
| Education (educated), n (%) | 39 (72.22%) | 27 (65.85%) | 0.504 |
| Smoking n (%) | 42 (77.78%) | 33 (82.50%) | 0.573 |
| Drinking n (%) | 26 (49.06%) | 17 (43.59%) | 0.604 |
| Duration of disease (<5 years), n (%) | 22 (40.74%) | 13 (31.71%) | 0.366 |
| WBC (×109/L) | 7.75 ± 3.58 | 6.95 ± 2.70 | 0.248 |
| RBC (×1012/L) | 4.36 ± 0.45 | 4.24 ± 0.51 | 0.255 |
| Hemoglobin (g/L) | 132.15 ± 15.40 | 128.16 ± 15.28 | 0.225 |
| Platelets (×109/L) | 227.78 ± 81.02 | 217.82 ± 72.34 | 0.547 |
| Albumin (g/L) | 37.14 ± 3.05 | 34.38 ± 3.87 | <0.001 |
| Triglycerides (mmol/L) | 1.21 ± 0.59 | 0.87 ± 0.37 | 0.001 |
| TC (mmol/L) | 4.39 ± 0.83 | 4.00 ± 0.71 | 0.025 |
| LDL (mmol/L) | 2.76 ± 0.76 | 2.36 ± 0.64 | 0.010 |
| HDL (mmol/L) | 1.04 ± 0.26 | 1.14 ± 0.27 | 0.076 |
| BODE | 2.76 ± 1.90 | 5.18 ± 2.42 | <0.001 |
| GOLD I or II, n (%) | 18 (35.29%) | 5 (12.82%) | 0.015 |
| CAT | 16.69 ± 5.58 | 18.95 ± 6.34 | 0.068 |
| PO2 | 71.63 ± 10.74 | 73.38 ± 19.01 | 0.604 |
| FEV1 | 1.09 ± 0.43 | 0.77 ± 0.33 | <0.001 |
| FEV1% | 44.60 ± 13.11 | 31.52 ± 12.81 | <0.001 |
| HAD(A) | 1.46 ± 2.43 | 1.41 ± 2.40 | 0.923 |
| HAD(D) | 3.30 ± 2.73 | 3.37 ± 3.48 | 0.913 |
| PSQI | 7.40 ± 4.31 | 7.27 ± 4.02 | 0.883 |
| MFS | 40.38 ± 17.73 | 45.13 ± 17.34 | 0.200 |
| Cognitive function (normal), n (%) | 35 (64.81%) | 18 (43.90%) | 0.042 |
COPD, chronic obstructive pulmonary disease; BMI, body mass index; WBC, white blood cells; RBC, red blood cells; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BODE, BMI, airflow obstruction, dyspnea and exercise capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT, COPD assessment test; FEV1, forced expiratory volume in 1 second; FEV1%, FEV1 related to predicted value; HAD(A) and HAD(D), Hospital Anxiety and Depression scales; PSQI, Pittsburgh Sleep Quality Index; MFS, Morse Fall Scale.
Association between nutritional risk and cognitive function
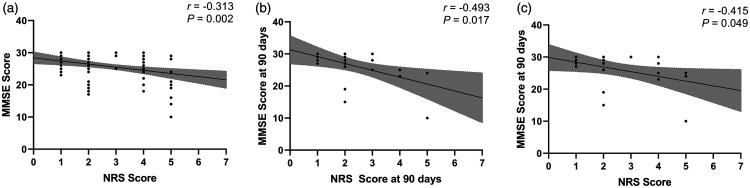
NRS 2002 scores were found to have a negative correlation with MMSE scores in our participants (Pearson r = −0.313, P = 0.002; Figure 1a).
Figure 1.
Correlation analyses between NRS 2002 and MMSE scores. (a) Correlation between NRS and MMSE scores at hospital admission, P = 0.002; (b) Correlation between NRS and MMSE scores at 90 days of follow-up, P = 0.017; (c) Correlation between NRS score at hospital admission and MMSE score at 90 days, P = 0.049.
NRS 2002, Nutritional Risk Screening 2002; MMSE, Mini-Mental State Examination.
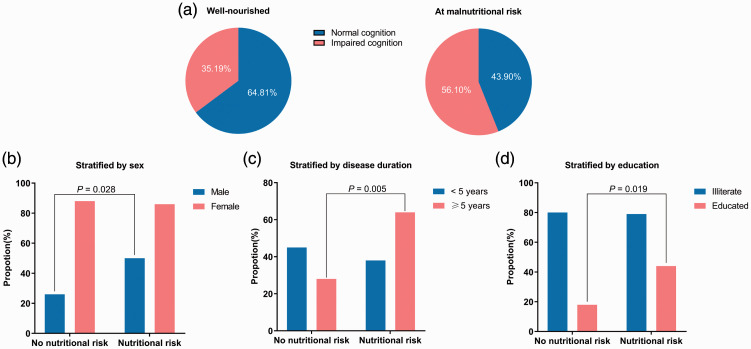
As shown in Figure 2a, fewer patients had normal cognition in the group at risk of malnutrition (43.90%) than the well-nourished group (64.81%, P = 0.042). We performed subgroup analysis to examine the association between nutritional risk and cognitive function according to sex, duration of disease, and level of education. A higher percentage of cognitively impaired patients in the malnutrition risk group was observed in male participants (P = 0.028) or those with longer duration of disease (≥5 years, P = 0.005) and with higher education levels (P = 0.019). However, in female participants or those with shorter duration of disease (<5 years) and less or no education, there was no significant association between cognitive function and nutritional status (Figure 2b, c, d).
Figure 2.
Impaired cognition in patients with COPD with different nutritional status in subgroup analyses. (a) Cognitive function in patients with COPD with different nutritional status. (b) Stratified by sex. (c) Stratified by disease duration. (d) Stratified by education.
COPD, chronic obstructive pulmonary disease.
Patients with nutritional risk and cognitive impairment
To gain a deeper understanding of the relationship between a risk of malnutrition and cognitive impairment, we performed univariate and multivariate logistic regression analyses. Based on univariate analysis, risk of malnutrition (P = 0.044), age (P = 0.006), sex (P = 0.002), education (P < 0.001), smoking (P = 0.019), albumin (P = 0.032), and MFS scores (P = 0.033) were all found to be significantly correlated with cognitive impairment in patients with COPD (Table 2).
Table 2.
Univariate logistic regression analyses of factors in cognitive function.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.082 | 1.023–1.143 | 0.006 |
| Sex, male | 0.087 | 0.018–0.415 | 0.002 |
| BMI | 0.942 | 0.838–1.060 | 0.320 |
| Educated | 0.105 | 0.037–0.300 | <0.001 |
| No smoking | 3.637 | 1.242–10.651 | 0.019 |
| No drinking | 2.158 | 0.923–5.043 | 0.076 |
| Duration of disease <5 years | 0.917 | 0.395–2.125 | 0.839 |
| WBC | 0.996 | 0.875–1.133 | 0.952 |
| RBC | 0.516 | 0.208–1.281 | 0.154 |
| Hemoglobin | 0.977 | 0.949–1.005 | 0.101 |
| Platelets | 1.005 | 0.999–1.010 | 0.116 |
| Albumin | 0.868 | 0.762–0.988 | 0.032 |
| Triglycerides | 0.819 | 0.369–1.814 | 0.622 |
| TC | 0.810 | 0.479–1.371 | 0.432 |
| LDL | 0.694 | 0.386–1.248 | 0.222 |
| HDL | 1.827 | 0.382–8.741 | 0.451 |
| BODE | 1.080 | 0.908–1.284 | 0.387 |
| GOLD I or II | 1.071 | 0.412–2.789 | 0.888 |
| CAT | 1.023 | 0.956–1.096 | 0.508 |
| PO2 | 0.970 | 0.940–1.001 | 0.061 |
| FEV1 | 0.423 | 0.140–1.274 | 0.126 |
| FEV1% | 1.002 | 0.973–1.032 | 0.878 |
| HAD (A) | 1.018 | 0.860–1.205 | 0.834 |
| HAD (D) | 1.137 | 0.990–1.307 | 0.070 |
| PSQI | 0.968 | 0.876–1.069 | 0.516 |
| MFS | 1.028 | 1.002–1.054 | 0.033 |
| Malnutrition risk | 2.354 | 1.024–5.409 | 0.044 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; WBC, white blood cells; RBC, red blood cells; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BODE, BMI, airflow obstruction, dyspnea and exercise capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FEV1%, FEV1 related to predicted value; HAD(A) and HAD(D), Hospital Anxiety and Depression scales; PSQI, Pittsburgh Sleep Quality Index; MFS, Morse Fall Scale.
Based on multivariate analysis, we found that malnutrition risk was independently and significantly associated with cognitive impairment. In model 1, relative to well-nourished patients, those who were at risk of malnutrition were significantly more likely to be cognitively impaired (odds ratio [OR] = 2.354, 95% confidence interval [CI]: 1.024–5.409, P = 0.044). After adjusting for age, sex, and duration of disease (model 2), the association between nutritional risk and cognitive impairment remained significant, with OR 3.472 (95% CI: 1.283–9.394, P = 0.014). Subsequent adjustment for age, sex, duration of disease, education, smoking history, albumin, and MFS scores (model 3) increased the OR to 4.120 (95% CI: 1.072–15.837, P = 0.039). These results indicate that malnutrition risk is an independent risk factor of cognitive impairment, and patients with COPD who are at risk of malnutrition are more likely to have cognitive impairment (Table 3).
Table 3.
Adjusted ORs (95% CIs) for cognitive function.
| OR | 95% CI | P value | |
|---|---|---|---|
| Model 1 | 2.354 | 1.024–5.409 | 0.044 |
| Model 2 | 3.472 | 1.283–9.394 | 0.014 |
| Model 3 | 4.120 | 1.072–15.837 | 0.039 |
Model 1 is univariate analysis. Model 2 is adjusted for age, sex, and duration of disease. Model 3 is adjusted for age, sex, duration of disease, education, smoking history, albumin, and MFS scores.
OR, odds ratio; CI, confidence interval.
Results in follow-up
To clarify the relationship between malnutrition risk and cognitive impairment over a long period, 23 patients with COPD were followed up for 90 days. We found a similar association between NRS 2002 and MMSE scores at 90 days of follow-up using a correlation test (Pearson r = −0.493, P = 0.017; Figure 1b) and logistic regression analysis (OR = 7.333, 95% CI: 1.114–48.264, P = 0.038; Table 4). We also performed a correlation analysis of NRS 2002 scores at hospital admission and MMSE scores at 90 days (Pearson r = −0.415, P = 0.049; Figure 1c), which suggested the predictive power of NRS 2002 scores for MMSE scores over a long period.
Table 4.
Univariate logistic regression analysis of nutritional status for cognitive function of follow-up.
| Variable |
Univariate logistic regression |
||
|---|---|---|---|
| OR | 95% CI | P value | |
| Malnutrition risk | 7.333 | 1.114–48.264 | 0.038 |
OR, odds ratio; CI, confidence interval.
Discussion
COPD is a progressive disease with a succession of comorbidities. Cognitive impairment as well as nutritional problems are considered frequent comorbidities owing to their high prevalence. López-Torres et al.12 reported the 48% of patients with COPD had impaired cognitive function according to the Montreal Cognitive Assessment. Parallel results were found by Schure et al.,20 with a proportion of nearly 30%, and by Yohannes et al.4 with 32%. Other studies have found a similarly high prevalence of malnutrition, ranging from 17% to 20%.21,22 Several researchers have focused on this high incidence of cognitive impairment and malnutrition among these patients, and some progress has been made regarding evidence of the relationship between nutritional risk and cognitive function. Metabolic causes may facilitate cognitive dysfunction in patients with diabetes, Alzheimer disease, and epilepsy.23–25 However, few studies have focused on the association between nutritional risk and cognitive function in patients with COPD.
Our research revealed a robust correlation between nutritional status and cognitive function in patients with COPD, especially in male individuals, patients with higher education levels, and those with a longer duration of disease. In multivariate logistic regression analysis, after taking into account covariates such as age, sex, duration of disease, education, smoking history, albumin, and MFS scores, we observed that NRS 2002 scores were independently associated with MMSE scores and patients with COPD with a nutritional risk were more likely to have cognitive impairment. Analogous results were found in an older Taiwanese population with hip fracture.26
Previous findings regarding the potential mechanism underlying how malnutrition influences cognitive function provided a basis for our findings, including the following. 1) Protein insufficiency. Albumin is a useful biochemical indicator of malnutrition.27 Thus, malnutrition signifies a lower level of serum albumin. Xu et al.28 deemed that a protein-rich dietary pattern was significantly associated with higher cognitive global scores and verbal memory scores. We obtained similar findings in our study; patients with COPD at risk of malnutrition had a lower level of albumin, and albumin was positively correlated with cognitive function (Table 2). 2) Micronutrient deficiency. According to a scoping review, selected micronutrients in adequate doses, including omega-3 fatty acids, potassium, and vitamins B6, B12, C, D, and E might play an ancillary role in improving cognitive function in older people.29 3) Inflammation. Bourassa and Sbarra30 argued that systemic inflammation is one biologically plausible mechanism through which body mass might influence cognitive function in aging adults. 4) Glucose supply. The vast majority of energy used by brain cells comes from blood glucose, which is involved in the transfer of energy from foods to neurons fundamental to the control of brain function.31,32
In our study, during follow-up, we still observed a link between nutritional risk and cognitive function, which implied that malnutrition contributes to cognitive impairment over a long period.
As far as we know, the present study is the first to identify an association between nutritional risk as evaluated by the NRS 2002, and cognitive function as evaluated by the MMSE, in patients with COPD. Although the detailed mechanisms behind this relationship remain to be elucidated, several potential mechanisms of pathogenesis have been suggested. Our research findings may help to guide clinical practice in the future. Nutritional intervention should be performed in randomized controlled trials, to verify its effect in improving cognitive impairment among patients with COPD.
There are some limitations in this study. Most of our study findings are based on the results of questionnaires, which may include information bias. Some patients may have problems with recall owing to poor memory, which would introduce bias in the results of scales based on self-assessment. Only 23 patients were followed up, which is a relatively small cohort and may lead to overrating or underestimation of the association between nutritional risk and cognitive function over time.
Conclusion
Patients with COPD at nutritional risk are more likely to have cognitive impairment. Nutritional status may predict cognitive function in patients with COPD to some extent; however, further studies are needed for confirmation. Specific and detailed evaluation of nutritional status are an important stage in the assessment of older patients with COPD, to prevent the onset of cognitive impairment.
Quick Look
Current knowledge
A host of studies have proved the important role of nutritional status in cognitive function in many diseases, such as Alzheimer disease and Parkinson disease. However, the relationship between nutritional status and cognitive function in patients with COPD has not been clarified.
What this paper contributes to our knowledge
NRS 2002 scores had a negative correlation with MMSE scores in patients with COPD. Participants with COPD at nutritional risk were more likely to have cognitive impairment, especially male patients, those with longer duration of disease (≥5 years), and those with higher education levels. Malnutrition risk was an independent risk factor for cognitive impairment in patients with COPD, after adjusting for several confounders. A similar association between NRS 2002 and MMSE scores was also found at 90 days of follow-up.
Acknowledgements
We would like to thank all the participants in the study and the staff at the Third Affiliated Hospital of Wenzhou Medical University for their contribution in obtaining the data and assisting in the successful completion of this study.
Authors’ contributions
YH and XZ formulated the research question and designed the study. XC, CL, HM, and RZ collected the data. XM, FF, JW, and KD organized the data. JM analyzed the data. JM, BJ, and NY wrote and reviewed the manuscript. All authors read and approved the final manuscript.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The present study was supported by the Wenzhou Municipal Sci-Tech Bureau Program (Y20180365).
ORCID iD: Xiaodiao Zhang https://orcid.org/0000-0002-1197-3276
References
- 1.Wen XH, Li Y, Han D, et al. The relationship between cognitive function and arterial partial pressure O2 in patients with COPD: A meta-analysis. Medicine 2018; 97: e9599. DOI: 10.1097/MD.0000000000009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelgrim CE, Peterson JD, Gosker HR, et al. Psychological co-morbidities in COPD: Targeting systemic inflammation, a benefit for both? Eur J Pharmacol 2019; 842: 99–110. DOI: 10.1016/j.ejphar.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Baird C, Lovell J, Johnson M, et al. The impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: A systematic review. Respir Med 2017; 129: 130–139. DOI: 10.1016/j.rmed.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Yohannes AM, Chen W, Moga AM, et al. Cognitive Impairment in Chronic Obstructive Pulmonary Disease and Chronic Heart Failure: A Systematic Review and Meta-analysis of Observational Studies. J Am Med Dir Assoc 2017; 18: 451.e1–451.e11. DOI: 10.1016/j.jamda.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Huang Y, Fei GH. The evaluation of cognitive impairment and relevant factors in patients with chronic obstructive pulmonary disease. Respiration 2013; 85: 98–105. DOI: 10.1159/000342970. [DOI] [PubMed] [Google Scholar]
- 6.Aras YG, Tunc A, Gungen BD, et al. The effects of depression, anxiety and sleep disturbances on cognitive impairment in patients with chronic obstructive pulmonary disease. Cogn Neurodyn 2017; 11: 565–571. DOI: 10.1007/s11571-017-9449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonelli Incalzi R, Marra C, Giordano A, et al. Cognitive impairment in chronic obstructive pulmonary disease–a neuropsychological and spect study. J Neurol 2003; 250: 325–332. DOI: 10.1007/s00415-003-1005-4. [DOI] [PubMed] [Google Scholar]
- 8.Canevelli M, Lucchini F, Quarata F, et al. Nutrition and Dementia: Evidence for Preventive Approaches? Nutrients 2016; 8: 144. DOI: 10.3390/nu8030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shatenstein B, Kergoat M, Reid I, et al. Dietary intervention in older adults with early-stage Alzheimer dementia: early lessons learned. J Nutr Health Aging 2008; 12: 461–469. DOI: 10.1007/BF02982707. [DOI] [PubMed] [Google Scholar]
- 10.Arie L, Herman T, Shema-Shiratzky S, et al. Do cognition and other non-motor symptoms decline similarly among patients with Parkinson's disease motor subtypes? Findings from a 5-year prospective study. J Neurol 2017; 264: 2149–2157. DOI: 10.1007/s00415-017-8605-x. [DOI] [PubMed] [Google Scholar]
- 11.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195: 557–582. DOI: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Torres I, Valenza MC, Torres-Sanchez I, et al. Changes in Cognitive Status in COPD Patients Across Clinical Stages. COPD 2016; 13: 327–332. DOI: 10.3109/15412555.2015.1081883. [DOI] [PubMed] [Google Scholar]
- 13.Karamanli H, Ilik F, Kayhan F, et al. Assessment of cognitive impairment in long-term oxygen therapy-dependent COPD patients. Int J Chron Obstruct Pulmon Dis 2015; 10: 2087–2094. DOI: 10.2147/COPD.S88326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. DOI: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 15.Mirza S, Clay RD, Koslow MA, et al. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin Proc 2018; 93: 1488–1502. DOI: 10.1016/j.mayocp.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Miravitlles M, Molina J, Quintano JA, et al. Depressive status explains a significant amount of the variance in COPD assessment test (CAT) scores. Int J Chron Obstruct Pulmon Dis 2018; 13: 823–831. DOI: 10.2147/COPD.S154791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Xin H, Yu J, et al. Abnormal intrinsic functional hubs and connectivity in stable patients with COPD: a resting-state MRI study. Brain Imaging Behav 2020; 14: 573–585. DOI: 10.1007/s11682-019-00130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orell-Kotikangas H, Osterlund P, Saarilahti K, et al. NRS-2002 for pre-treatment nutritional risk screening and nutritional status assessment in head and neck cancer patients. Support Care Cancer 2015; 23: 1495–1502. DOI: 10.1007/s00520-014-2500-0. [DOI] [PubMed] [Google Scholar]
- 19.Westergren A, Norberg E, Hagell P. Diagnostic performance of the Minimal Eating Observation and Nutrition Form - Version II (MEONF-II) and Nutritional Risk Screening 2002 (NRS 2002) among hospital inpatients - a cross-sectional study. BMC Nurs 2011; 10: 24. DOI: 10.1186/1472-6955-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schure MB, Borson S, Nguyen HQ, et al. Associations of cognition with physical functioning and health-related quality of life among COPD patients. Respir Med 2016; 114: 46–52. DOI: 10.1016/j.rmed.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Mete B, Pehlivan E, Gulbas G, et al. Prevalence of malnutrition in COPD and its relationship with the parameters related to disease severity. Int J Chron Obstruct Pulmon Dis 2018; 13: 3307–3312. DOI: 10.2147/COPD.S179609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Blasio F, Di Gregorio A, De Blasio F, et al. Malnutrition and sarcopenia assessment in patients with chronic obstructive pulmonary disease according to international diagnostic criteria, and evaluation of raw BIA variables. Respir Med 2018; 134: 1–5. DOI: 10.1016/j.rmed.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Riederer P, Korczyn AD, Ali SS, et al. The diabetic brain and cognition. J Neural Transm (Vienna) 2017; 124: 1431–1454. DOI: 10.1007/s00702-017-1763-2. [DOI] [PubMed] [Google Scholar]
- 24.Santos CY, Snyder PJ, Wu WC, et al. Pathophysiologic relationship between Alzheimer's disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst) 2017; 7: 69–87. DOI: 10.1016/j.dadm.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tedrus G, Srebernich SM, Santos TBN. Correlation between clinical and cognitive aspects and nutritional indicators of elderly patients with new-onset epilepsy. Epilepsy Behav 2018; 85: 105–109. DOI: 10.1016/j.yebeh.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 26.Wang HP, Liang J, Kuo LM, et al. Trajectories of Nutritional Status and Cognitive Impairment among Older Taiwanese with Hip Fracture. J Nutr Health Aging 2017; 21: 38–45. DOI: 10.1007/s12603-016-0756-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Pereira SL, Luo M, et al. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017; 9: 829. DOI: 10.3390/nu9080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Parker D, Shi Z, et al. Dietary Pattern, Hypertension and Cognitive Function in an Older Population: 10-Year Longitudinal Survey. Front Public Health 2018; 6: 201. DOI: 10.3389/fpubh.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iolascon G, Gimigliano R, Bianco M, et al. Are Dietary Supplements and Nutraceuticals Effective for Musculoskeletal Health and Cognitive Function? A Scoping Review. J Nutr Health Aging 2017; 21: 527–538. DOI: 10.1007/s12603-016-0823-x. [DOI] [PubMed] [Google Scholar]
- 30.Bourassa K, Sbarra DA. Body mass and cognitive decline are indirectly associated via inflammation among aging adults. Brain Behav Immun 2017; 60: 63–70. DOI: 10.1016/j.bbi.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 2008; 9: 568–578. DOI: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu CC, Wahlqvist ML, Lee MS, et al. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 2011; 24: 485–493. DOI: 10.3233/JAD-2011-101524. [DOI] [PubMed] [Google Scholar]