Figure 1.
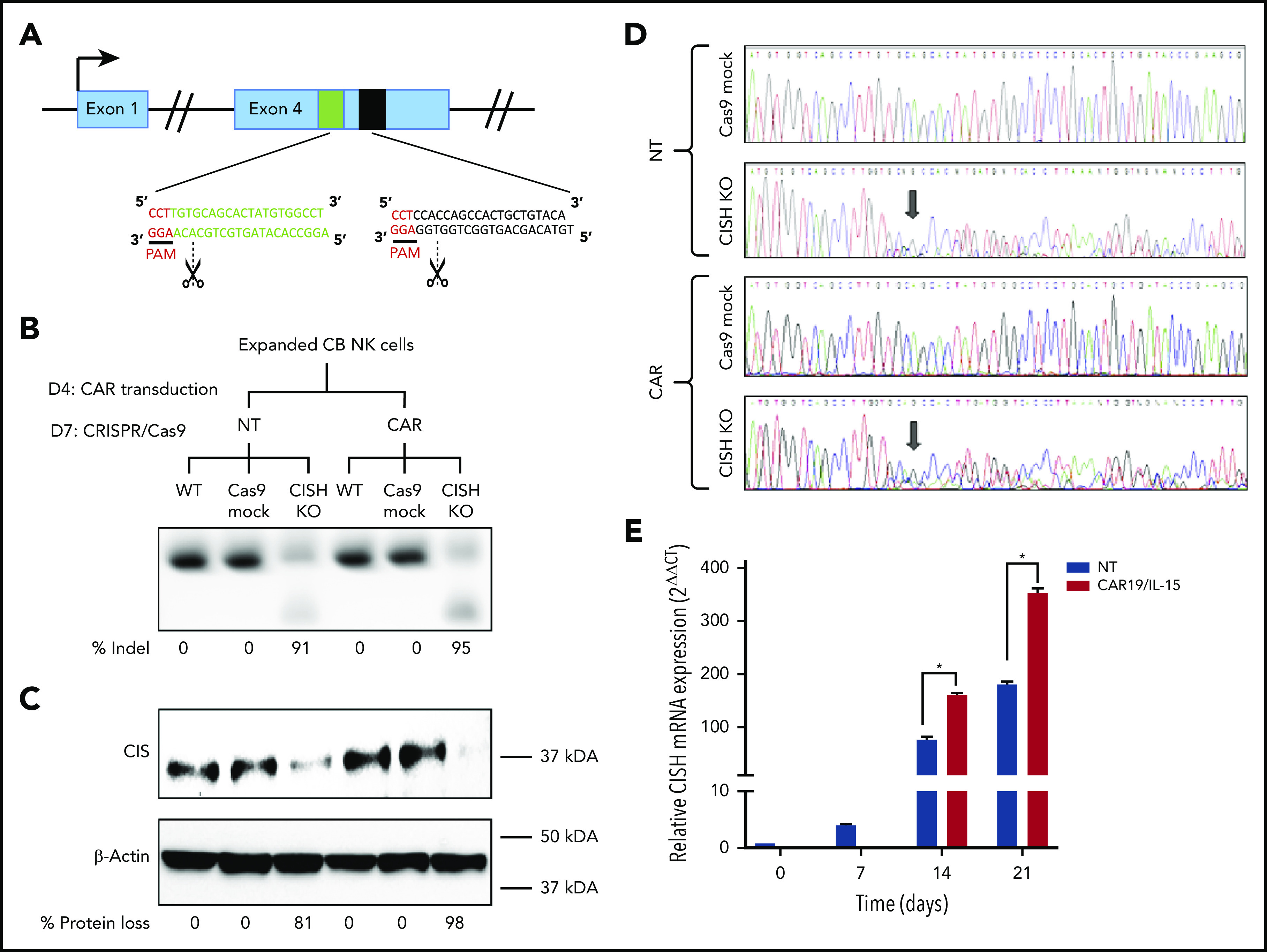
CRISPR-Cas9–mediated deletion of CISH in iC9/CAR19/IL-15 NK cells. (A) Schematic representation of CRISPR-Cas9–mediated CISH KO using 2 guide RNAs (gRNA) targeting exon 4 of the CISH gene. PAM, protospacer-adjacent motif. (B-C) CB-NK cells were expanded with K562 based feeder cells and IL-2 and then either left NT or transduced with a retroviral vector expressing iC9/CAR19/IL-15 construct on day 4 (D4) of expansion. On day 7 (D7) of expansion, NT and iC9/CAR19/IL-15–expressing CB-NK cells were nucleofected with Cas9 alone (Cas9 mock), Cas9 preloaded with gRNA targeting CISH exon 4 (CISH KO), or nonnucleofected (wild-type [WT]). The CISH KO efficiency was determined by PCR (B) and western blot analysis (C). (D) Sanger sequencing results showing multiple peaks reflecting nonhomologous end-joining (NHEJ) events in NT or iC9/CAR19/IL-15 (CAR) NK cells that underwent CISH KO compared with single peaks in control (Cas9 mock). Arrows indicate the base pair position where the gene editing started. (E) Bar graphs showing the relative mRNA expression levels of CISH determined on days 0, 7, 14, and 21 of expansion in NT (blue) and iC9/CAR19/IL-15 (red) NK cells by reverse transcription polymerase chain reaction (RT-PCR) (n = 3). Note that on days 0 and 7 only data for NT-NK cells are included since the CAR transduction step is performed on day 4 of expansion. 18 S ribosomal RNA (18S) was used as the internal reference gene. Bars represent mean values with standard deviation, *P ≤ .05.
