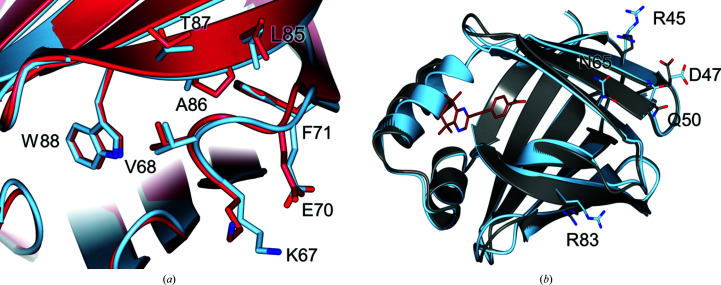
Figure 8.
(a) Least-squares superposition of H. sapiens CRABPI-L29C–DC645 (chain A, pale blue) and M. musculus CRABPI (PSB entry 1cbr, crimson), highlighting the β-strand region residues 70–90 where the key P86A modification that differentiates the proteins is found. The r.m.s.d of the two structures on Cα atoms is 0.69 Å. (b) Alignment of unoccupied (chain B, grey) and ligand-occupied (chain A, pale blue) monomers of CRABPI-L29C–DC645, showing differences between the two forms (r.m.s.d. of 0.66 Å on Cα atoms).