Abstract
Autism Spectrum Disorder (ASD) symptom emergence is heterogeneous, yet literature comparing young children diagnosed early versus later is relatively scant. Toddlers diagnosed with ASD between 12–18 months (n = 20), 19–24 months (n = 65), or 25–41 months (n = 27) were compared on demographics, developmental functioning, and symptoms. Later diagnosed children were more impaired than both younger groups on nonverbal reasoning, adaptive behavior, and ASD severity. Fine motor, receptive language, and social skills followed a linear pattern, with 25–41M children more delayed than 19–24M participants, who were more delayed than 12–18M toddlers. Communication impairments did not differ among groups. Universal ASD screening before 18 months may detect toddlers when symptoms are milder and more amenable to intervention.
Keywords: autism spectrum disorder, early identification, early diagnosis, later diagnosis, symptom onset
Lay Abstract
What is already known about the topic?
The emergence of autism symptoms in childhood is variable, with some children showing signs of Autism Spectrum Disorder (ASD) very early, and others not being identified until much later. Though most children in the United States are not diagnosed with ASD until preschool, at ages three to four years, symptoms can be reliably detected at 14 months. It is less certain how those toddlers diagnosed with ASD earlier versus later differ from each other clinically.
What this paper adds?
This study revealed that young children diagnosed later in development, between ages 25 and 41 months, are more impaired on measures of cognitive, adaptive, and social functioning than their counterparts who are diagnosed with ASD earlier. All young children with ASD are impaired in communication to a similar degree, however.
Implications for practice, research, or policy.
Universal autism screening at 18 months may identify toddlers with ASD when their symptoms are milder and more readily amenable to intervention. Repeated screening at 24 months is supported to detect those children missed by an earlier screening, who may be more severely affected. Caregivers should be encouraged to pursue diagnostic evaluation at an initial positive screening result to ensure timely diagnosis and treatment.
The emergence of Autism Spectrum Disorder (ASD) symptoms in the first two years of life is well-documented; in fact, many toddlers who are later diagnosed with ASD exhibit behavioral and neurological markers by their first birthdays (Hazlett et al., 2017; Martinez-Pedraza & Carter, 2009; Osterling, Dawson, & Munson, 2002; Ozonoff et al., 2010). Even so, most children are identified after age three (Centers for Disease Control and Prevention [CDC], 2018). ASD diagnoses are considered stable by 18 to 24 months of age (Ozonoff et al., 2015), consistent with American Academy of Pediatrics [AAP] recommendations for ASD-specific screening at those ages (Duby et al., 2006; Johnson & Myers, 2007). Recent evidence suggests that ASD can be reliably diagnosed even earlier; Pierce and colleagues (2019) found diagnostic stability of ASD to be 0.79 by 14 months, higher than for language disorder (0.14) and global developmental delay (0.44).
With the increased focus on early detection and intervention for ASD, there have also been enhanced efforts to understand the emergence of ASD symptoms and developmental trajectories of children diagnosed at various ages. Historically, there were thought to be two major symptom onset patterns in ASD: (1) early onset of impairment and ASD symptomatology, without regression, and (2) a period of typical or mildly delayed development followed by a pronounced loss of social communication skills and the emergence of restricted and repetitive behaviors, usually between 18 and 24 months (Luyster et al., 2005). Alternatively, patterns of emergence were defined as early ASD diagnosis, manifested by 14 months of age, and later diagnosis, with later emergence of symptoms but before age three years (Landa, Holman, & Garrett-Mayer, 2007). More recently, conceptualizations of symptom onset have broadened to include developmental plateauing and “mixed features” (i.e., early symptom onset followed by a period of regression; Ozonoff et al., 2010). It seems apparent that a subset of children initially diagnosed with ASD improve moderately, whereas other individuals exhibit persistent and severe ASD symptoms, and still others display symptoms that worsen in the early developmental period (Kim et al., 2018; Lord, Luyster, Guthrie, & Pickles, 2012). Of note, results from recent, large-scale investigations of young children with ASD suggest that a large proportion, if not the majority, of those children demonstrate regression, with a loss of key social behaviors within the first two years of life (Ozonoff et al., 2018; Ozonoff & Iosif, 2019). The rates of regression in ASD may be underreported due to differences in measurement method, particularly problems with retrospective caregiver reports of loss of skills (Ozonoff et al., 2018). Therefore, a dimensional approach, in which onset of ASD symptoms is described in terms of the extent and timing of regression, might best account for the heterogeneity in ASD symptom emergence and developmental trajectory (Ozonoff et al., 2010).
Despite efforts to understand patterns of symptom onset and outcomes in children with ASD, limited literature exists regarding toddlers diagnosed with ASD before age 18 months, particularly compared to those diagnosed later. Thus, it is unclear if toddlers diagnosed with ASD early versus later represent unique phenotypes of the disorder, with potentially distinct developmental trajectories and prognoses, or if differences in age at diagnosis are instead due to screening and diagnostic practices or other child and family characteristics. To date, primarily studies of infant siblings of ASD probands have investigated the early development of social and communication skills by comparing those diagnosed with ASD early (i.e., by age 14 months) to children diagnosed later (i.e., by 30 to 36 months of age; Landa et al., 2007; Landa, Gross, Stuart, & Faherty, 2013). The early diagnosed children exhibited earlier disruption in social and language development, contributing to the timing of their ASD diagnoses, yet communication, socialization, and play behaviors were similar to later diagnosed children by age 24 months. Of note, later diagnosed children displayed a “preclinical phase” prior to their ASD diagnoses: they were mildly impaired in language and fine motor skills at 14 months yet did not meet diagnostic criteria for ASD (Landa et al., 2013). Consistent with a dimensional approach to symptom onset, it is possible that later diagnosed toddlers demonstrate mild impairment, perhaps a prodromal ASD presentation, very early in neurodevelopment, and they then undergo a developmental shift and decline between 14 and 24 months of age (Landa et al., 2007; Landa et al., 2013).
Early intensive behavioral intervention (EIBI) for ASD, particularly when initiated before age three years, can contribute to marked improvement in symptoms and overall development. Young children who participate in EIBI demonstrate larger gains in intellectual and language functioning compared to peers with ASD who do not engage in early intervention (Anderson, Liang, & Lord, 2014; Harris & Handleman, 2000; MacDonald, Parry-Cruwys, Dupere, & Ahearn, 2014; Rogers & Vismara, 2008). Thus, it is essential to understand ASD symptom development and emergence in the first three years, and to clarify whether toddlers diagnosed early or later in that developmental period represent distinct ASD phenotypes that may differentially benefit from intervention services. Given the current knowledge, if ASD diagnoses occurred closer to the first birthday, children with ASD might exhibit enhanced improvement in response to EIBI (Landa et al., 2007; Landa et al., 2013; MacDonald et al., 2014). On the other hand, if the earliest diagnosed children are detected earlier because their developmental lags are more severe and thus more apparent at a young age, their response to EIBI might be tempered by the severity of their impairment, as prior research on very low-functioning toddlers with ASD has shown that these children exhibit limited developmental gains over time despite provision of targeted intervention services (Hinnebusch, Miller, & Fein, 2017).
The present study aims to better understand the emergence of symptoms, particularly potential differences in symptom presentation and developmental functioning, based on timing of diagnosis. Specifically, we examined toddlers who attended pediatric practices that were randomized to begin ASD-specific screening between 12 and 18 months as part of a general population-based screening study. Although all children in our sample were not screened at every time point given this study design, every participant was screened for ASD by his or her 18-month well-child care visit. Participating toddlers were then divided into groups based on diagnostic age bracket (i.e., 12–18 months, 19–24 months, or 25–41 months) and compared on demographic and family characteristics, cognitive development, and ASD severity. Although Landa et al. (2013) did not find full support for early diagnosed children having less promising short-term prognoses, as those children appeared similar to later diagnosed toddlers by age 24 months, they documented more pronounced delays in language and social functioning in early-versus later-manifesting ASD at initial diagnosis. Therefore, we hypothesized that our early diagnosed group would exhibit more significant developmental delays than those diagnosed later in development. Further, although universal screening decreases disparities in age at diagnosis for minority children (Herlihy et al., 2014), we predicted that lower socioeconomic status would be associated with later ASD diagnosis since previous research has documented that children from less economically advantaged backgrounds are diagnosed later (Mandell, Novak, & Zubritsky, 2005). We intend to add to the current understanding of symptom onset in ASD, particularly relating to early diagnosis, in order to promote detection and treatment of ASD even sooner in development.
Methods
Participants
Participants were 112 toddlers with ASD from a multi-site investigation of the early detection of ASD, which recruited from pediatric practices in Connecticut, metropolitan Atlanta, and metropolitan Philadelphia. All participants screened positive on a validated ASD-specific screening instrument at a pediatric well-child care visit between the ages of 11 months, 15 days and 38 months, 30 days, with initial screening and study enrollment occurring no later than 21 months, 30 days (i.e., the upper age range of our 18-month screening time point). Children were excluded from this study if they were inadvertently screened outside of the larger study’s age range (n = 2); if they received another developmental diagnosis prior to being diagnosed with ASD (n = 4); or if they were first diagnosed with ASD before 18 months but no longer met diagnostic criteria at a later confirmatory evaluation (n = 1).
Participants were classified into three groups based on their age at ASD diagnosis: 12–18M, 19–24M, and 25–41M; see Table 1 for demographics. These groupings allow comparison of children diagnosed early (prior to the AAP’s recommended 18-month screening) to those diagnosed by age 2 years (following 18-month screening, at which time the majority of children with ASD are identified in our sample [see Dai et al., 2019 and Duby et al., 2006], referred to as our “middle” group), as well as to toddlers diagnosed later (either after the AAP’s recommended second 24-month screening time, which is important to identify children missed at an earlier screening [see Dai et al., 2019 and Gupta et al., 2007], or due to delays between positive screening and evaluation).
Table 1.
Demographic Characteristics by Diagnostic Age Bracket
| Variable | 12–18M (n = 20) | 19–24M (n = 65) | 25–41M (n = 27) | p |
|---|---|---|---|---|
| Child age at initial positive screening in months [M (SD)] | 13.0 (1.2) | 18.5 (2.5) | 22.1 (4.4) | < .001 |
| Child age at evaluation in months [M (SD)] | 15.6 (1.8) | 21.1 (1.6) | 30.1 (4.7) | < .001 |
| Child sex [N (%)] | ||||
| Male | 15 (75.0) | 47 (72.3) | 23 (85.2) | .443 |
| Female | 5 (25.0) | 18 (27.7) | 4 (14.8) | |
| Child race [N (%)] | ||||
| White | 9 (47.4) | 32 (52.5) | 9 (37.5) | |
| Black | 4 (21.1) | 15 (24.6) | 3 (12.5) | |
| Asian | 2 (10.5) | 6 (9.8) | 6 (25.0) | .441 |
| American Indian | 1 (5.3) | 1 (1.6) | 1 (4.2) | |
| Biracial | 3 (15.8) | 7 (11.5) | 5 (20.8) | |
| Child ethnicity [N (%)] | ||||
| Not Hispanic | 13 (68.4) | 29 (58.0) | 16 (64.0) | .999 |
| Hispanic | 6 (31.6) | 21 (42.0) | 9 (36.0) | |
| Language exposure [N (%)] | ||||
| Monolingual | 11 (57.9) | 31 (57.4) | 9 (45.0) | .654 |
| Multilingual | 8 (42.1) | 23 (42.6) | 11 (55.0) | |
| Maternal age in years [M (SD)] | 29.9 (5.7) | 30.4 (5.2) | 30.7 (6.0) | .865 |
| Maternal race [N (%)] | ||||
| White | 10 (52.6) | 32 (58.2) | 10 (45.5) | |
| Black | 4 (21.1) | 16 (29.1) | 4 (18.2) | |
| Asian | 4 (21.1) | 5 (9.1) | 7 (31.8) | .185 |
| American Indian | 1 (5.3) | 2 (3.6) | 0 | |
| Biracial | 0 | 0 | 1 (4.5) | |
| Maternal ethnicity [N (%)] | ||||
| Not Hispanic | 12 (70.6) | 38 (70.4) | 15 (71.4) | .972 |
| Hispanic | 5 (29.4) | 16 (29.6) | 6 (28.6) | |
| Maternal education [N (%)] | ||||
| No degree | 2 (10.0) | 4 (6.3) | 0 | |
| High school diploma/GED | 6 (30.0) | 27 (42.2) | 11 (42.3) | |
| Some college | 4 (20.0) | 11 (17.2) | 8 (30.8) | .529 |
| College degree | 4 (20.0) | 9 (14.1) | 5 (19.2) | |
| Post-college degree | 4 (20.0) | 13 (20.3) | 2 (7.7) | |
| Paternal age in years [M (SD)] | 31.4 (7.2) | 33.3 (6.4) | 32.7 (5.9) | .546 |
| Paternal race [N (%)] | ||||
| White | 8 (50.0) | 32 (64.0) | 9 (40.9) | |
| Black | 5 (31.3) | 13 (26.0) | 7 (31.8) | .470 |
| Asian | 2 (12.5) | 4 (8.0) | 5 (22.7) | |
| American Indian | 0 | 1 (2.0) | 1 (4.5) | |
| Biracial | 1 (6.3) | 0 | 0 | |
| Paternal ethnicity [N (%)] | ||||
| Not Hispanic | 12 (70.6) | 37 (72.5) | 15 (68.2) | .980 |
| Hispanic | 5 (29.4) | 14 (27.5) | 7 (31.8) | |
| Paternal education [N (%)] | ||||
| No degree | 1 (5.9) | 2 (4.2) | 1 (4.8) | |
| High school diploma/GED | 10 (58.8) | 17 (35.4) | 9 (42.9) | |
| Some college | 1 (5.9) | 8 (16.7) | 5 (23.8) | .671 |
| College degree | 2 (11.8) | 13 (27.1) | 4 (19.0) | |
| Post-college degree | 3 (17.6) | 8 (16.7) | 2 (9.5) | |
| Annual income [N (%)] | ||||
| $12,000 or less | 2 (25.0) | 9 (14.8) | 5 (22.7) | |
| $12,001-$24,000 | 5 (27.8) | 7 (11.5) | 2 (9.1) | |
| $24,001-$36,000 | 1 (5.6) | 11 (18.0) | 1 (4.5) | |
| $36,001-$48,000 | 1 (5.6) | 2 (3.3) | 0 | |
| $48,001-$60,000 | 0 | 4 (6.6) | 4 (18.2) | .251 |
| $60,001-$72,000 | 1 (5.6) | 5 (8.2) | 0 | |
| $72,001-$84,000 | 0 | 3 (4.9) | 3 (13.6) | |
| $84,001-$96,000 | 1 (5.6) | 1 (1.6) | 1 (4.5) | |
| $96,001 or more | 7 (38.9) | 19 (31.1) | 6 (27.3) | |
| Family ASD history [N (%)] | ||||
| Positive | 3 (25.0) | 9 (20.9) | 3 (17.6) | .849 |
| Negative | 9 (75.0) | 34 (79.1) | 14 (82.4) |
Note. Demographic and family data are presented for participants with complete data, and all reported percentages were calculated using the number of participants for whom completed data was available for that specific variable as denominators.
Procedures
Participants were screened at their pediatrician’s office or remotely using an online platform created for the screening study. Participating community pediatric sites were randomized to one of three screening schedules: (a) 12, 18, 24, and 36 months; (b) 15, 18, 24, and 36 months; or (3) 18, 24, and 36 months (see Figure 1). While sites were encouraged to enroll toddlers at their site’s specific initial screening time (i.e., 12, 15, or 18 months), enrollment was allowed at all sites until age 21 months, 30 days, the upper end of the 18-month screening window, to increase recruitment and account for caregiver and provider reluctance to screen for ASD at earlier ages. At 12 months (11 months, 15 days to 13 months, 30 days), children were screened with the Infant/Toddler Checklist (ITC; Wetherby & Prizant, 2001) and the First Year Inventory – Lite (FYI-L; Baranek, Watson, Crais, Turner-Brown & Reznick, 2013). At 15 months (14 months to 16 months, 30 days), participants were screened with the FYI-L and the Modified Checklist for Autism in Toddlers, Revised with Follow-Up (M-CHAT-R/F; Robins et al., 2014). At 18 months (17 months to 21 months, 30 days), children were (re-)screened with the M-CHAT-R/F. The M-CHAT-R/F was also administered to all participants at 24 months (22 months to 29 months, 30 days) and 36 months (34 months to 38 months, 30 days).
Figure 1.
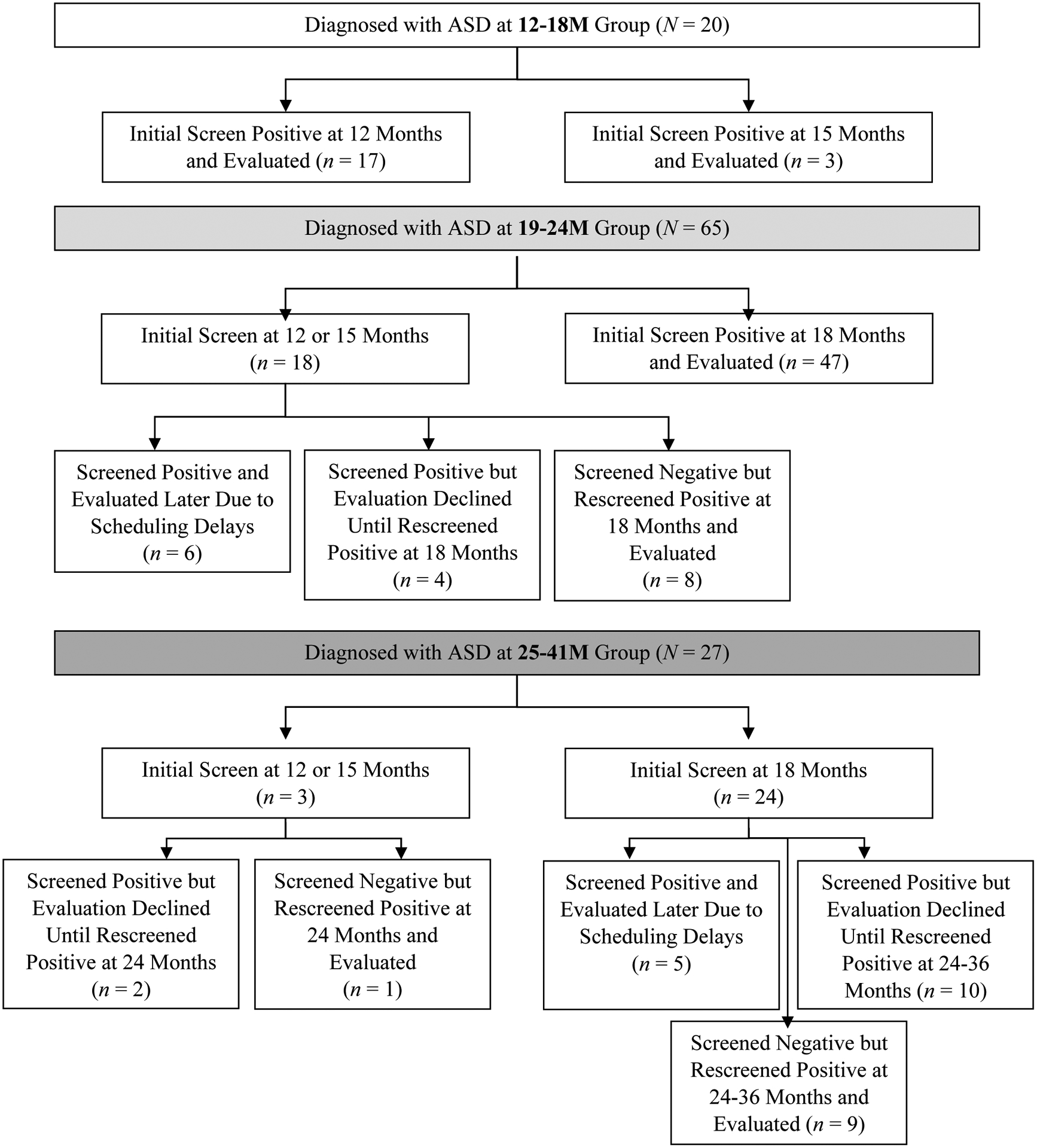
Screening schedules and outcomes by age group. Within each diagnostic age bracket (i.e., 12–18M, 19–24M, 25–41M), participant screening trajectories are presented. Per the study protocol, children were screened through their pediatrician’s offices, which were randomized to screen children initially at 12, 15, or 18 months. Rescreening occurred for all participants at 18, 24, and 36 months.
Caregivers of children who screened positive for ASD were called by the research team and offered a free diagnostic and developmental evaluation for their child. Evaluations were conducted by a licensed psychologist, certified school psychologist, or a developmental-behavioral pediatrician and a supervised doctoral student or research staff. Evaluations took place at the research team’s university clinics when possible, or at their pediatrician’s office. Diagnoses were based on clinical best estimate, incorporating child observation, developmental history, and direct testing. To meet study criteria for ASD, children had to meet ICD-10 diagnostic criteria for either Childhood Autism or Atypical Autism (World Health Organization [WHO], 2004), or DSM-5 diagnostic criteria for Autism Spectrum Disorder (APA, 2013). As evidence of the stability of ASD diagnoses prior to 18 months is still emerging, toddlers diagnosed before age 18 months were invited for confirmatory evaluations between 24 and 36 months.
This project was approved by the Institutional Review Boards (IRBs) at all participating institutions. Written informed consent was obtained from caregivers.
Measures
As screening instruments were not analyzed in the current study, they are not detailed.
Evaluation History Form.
The Evaluation History Form collected child and family demographics (i.e., race, ethnicity, any languages spoken by the family, parental education, family income, and family history of ASD or other neurodevelopmental concerns) and child developmental history. The History Form was completed by the child’s caregiver prior to the evaluation.
Mullen Scales of Early Learning (MSEL; Mullen, 1995).
The MSEL assesses cognitive, motor, and language abilities in children ages one to 68 months. The current study used the Visual Reception, Fine Motor, Receptive Language, and Expressive Language domains. MSEL T-scores were not normally distributed due to floor effects (i.e., T = 20). Thus, age-equivalent (AE) scores in each domain were converted to developmental quotient scores, as follows: [(AE ÷ chronological age) * 100] (Reitzel et al., 2013).
Vineland Adaptive Behavior Scales, Second Edition: Survey Interview Form (VABS-II; Sparrow, Cicchetti, & Balla, 2005).
The VABS-II is a semi-structured caregiver interview that assesses adaptive behaviors in the domains of Communication, Daily Living Skills, Socialization, and Motor Skills. Standard scores in each domain were used.
Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord, Luyster, Gotham, & Guthrie, 2012; Lord et al., 2012).
The ADOS-2 is a semi-structured observational tool designed to measure symptoms of ASD. The current study used the ADOS-2 Toddler Module, designed for preverbal children ages 12 to 30 months, for the majority of participants (Lord, Luyster et al., 2012). Module 1 was used for older preverbal toddlers (i.e., >30 months), and Module 2 was used for toddlers with flexible phrase speech (Lord, Rutter et al., 2012). Comparison scores (range: 1–10) reflect overall ASD severity (i.e., Total Score) and symptom severity in the domains of Social Affect and Restricted/Repetitive Behaviors (Esler et al., 2015; Hus, Gotham, & Lord, 2014).
Childhood Autism Rating Scale, Second Edition (CARS2; Schopler, Van Bourgondien, Wellman, & Love, 2010).
The CARS2 is a 15-item clinician rating scale that uses direct observation and caregiver report to assess ASD symptom severity. A total score, based on the sum of individual items, classifies a child as having minimal-to-no symptoms (total score = 15 – 29.5), mild-to-moderate symptoms (total score = 30 – 36.5), or severe symptoms of ASD (total score = 37 – 60).
Data Analytic Plan
We first examined whether diagnostic age bracket was associated with any demographic factors to determine whether those variables needed to be held constant in subsequent analyses assessing developmental functioning and ASD symptomatology. Categorical data were examined utilizing a series of Chi square tests of independence; in cases of small cell counts, Fisher’s exact test was used. Continuous variables were analyzed using one-way analysis of variance (ANOVA). Demographic variables that did not significantly differ by diagnostic age bracket were not included in further analyses.
We then compared our groups on cognitive and adaptive skills and ASD severity using a series of one-way ANOVAs. In cases of significant ANOVAs, post-hoc Tukey’s HSD Tests determined specific group differences.
All analyses were run using IBM SPSS Statistics for Mac, Version 26 (IBM Corp., 2019).
Results
Demographic and Family Factors
Groups were first compared on demographic and family factors to determine whether any of those variables were associated with diagnostic age bracket (see Table 1). Child sex (p = .443, Fisher’s Exact test, Cramer’s V = .125), child race (p = .441, Fisher’s Exact test, Cramer’s V = .183), child ethnicity (X2(2, N = 104) = .102, p = .999, Cramer’s V = .031), or the number of languages a child was exposed to at home (i.e., monolingual versus multilingual status; X2(2, N = 93) = .997, p = .654, Cramer’s V = .104) did not differ among age groups. Furthermore, age groups did not differ by maternal factors, including race (p = .185, Fisher’s Exact test, Cramer’s V = .238), ethnicity (p = .972, Fisher’s Exact test, Cramer’s V = .048), level of education (p = .529, Fisher’s Exact test, Cramer’s V = .176), or age (F(2, 103) = .145, p = .865, ηp2 = .003), nor did they differ based on any paternal factors, including race (p = .470, Fisher’s Exact test, Cramer’s V = .214), ethnicity (p = .980, Fisher’s Exact test, Cramer’s V = .051), education level (p = .671, Fisher’s Exact test, Cramer’s V = .181), or age (F(2, 97) = .609, p = .546, ηp2 = .012). Annual household income was also not associated with diagnostic age bracket (p = .251, Fisher’s Exact test, Cramer’s V = .310).
As the study sample was drawn from a general population-based screening sample rather than an infant sibling sample, we examined whether groups differed based on a family history of ASD. Fifteen participants in our sample reported a family history of ASD, but the proportion of families reporting this did not differ by age group (p = .849, Fisher’s Exact test, Cramer’s V = .923).
Since diagnostic age bracket was not associated with any of the measured demographic or family factors (Table 1), these factors were not included in subsequent analyses.
Developmental Functioning
One-way ANOVAs were run to determine whether diagnostic age bracket was associated with a child’s cognitive or adaptive functional level. As shown in Table 2, results indicated a significant main effect of diagnostic age bracket on MSEL Visual Reception (i.e., visual problem solving) abilities (F(2, 107) = 9.840, p < .001, ηp2 = .155), such that scores were significantly lower for children in the 25–41M group compared to those in the 12–18M (p = .001) and 19–24M (p < .001) groups, who did not differ from each other (p = .847).
Table 2.
Developmental Functioning by Diagnostic Age Bracket
| Variable | 12–18M (n = 20) | 19–24M (n = 65) | 25–41M (n = 27) | p |
|---|---|---|---|---|
| MSEL developmental quotient [M (SD)] | ||||
| Visual receptiona | 78.7 (22.4) | 75.6 (23.2) | 54.3 (17.9) | < .001 |
| Fine motorb | 96.0 (14.3) | 80.8 (19.4) | 60.1 (16.3) | < .001 |
| Receptive languagea | 58.4 (24.6) | 52.5 (25.9) | 38.7 (21.4) | .019 |
| Expressive languagec | 58.0 (18.4) | 51.5 (20.4) | 46.6 (21.2) | .177 |
| VABS-II standard score [M (SD)] | ||||
| Communicationc | 78.2 (12.8) | 74.5 (15.3) | 71.0 (10.9) | .219 |
| Daily living skillsa | 85.9 (13.2) | 85.5 (12.8) | 77.4 (10.3) | .013 |
| Socializationb | 88.0 (9.3) | 81.1 (9.6) | 73.6 (9.0) | < .001 |
| Motor skillsa | 91.3 (9.1) | 88.6 (12.2) | 81.9 (11.2) | .012 |
| ADOS-2 comparison score [M (SD)] | ||||
| Overall totalc | 7.25 (2) | 7.51 (1.83) | 8.37 (1.78) | .071 |
| Social affectc | 7.6 (1.98) | 7.78 (1.92) | 8.19 (1.82) | .536 |
| Restricted and repetitive behaviord | 6.20 (2.35) | 6.62 (1.97) | 7.85 (1.81) | .009 |
| CARS2 total score [M (SD)]d | 31.5 (8.4) | 32.4 (5.7) | 36.6 (5.7) | .007 |
Note. MSEL = Mullen Scales of Early Learning; VABS-II = Vineland Adaptive Behavior Scales, Second Edition; ADOS-2 = Autism Diagnostic Observation Schedule, Second Edition; CARS2 = Childhood Autism Rating Scale, Second Edition. MSEL and VABS-II standard scores have a mean of 100 and standard deviation of 15. ADOS-2 comparison scores range from 1 to 10 with higher scores indicating greater autism severity. CARS2 scores over 30 indicate that symptoms of autism are present, with higher scores suggesting greater autism severity.
On post-hoc analyses, 12–18M = 19–24M > 25–41M.
On post-hoc analyses, 12–18M > 19–24M > 25–41M.
On post-hoc analyses, 12–18M = 19–24M = 25–41M.
On post-hoc analyses, 12–18M = 19–24M < 25–41M.
Similarly, MSEL Fine Motor scores were significantly associated with diagnostic age bracket, with a large effect size (F(2, 107) = 23.25, p < .001, ηp2 = .303). Again, scores were much lower for children in the 25–41M group compared to those in the 12–18M (p < .001) and 19–25M (p < .001) groups. Fine motor skills were also significantly lower for toddlers diagnosed between ages 19 and 24 months (p = .004) compared to those diagnosed earlier (i.e., 12–18M group).
Results also revealed a significant main effect for diagnostic age bracket on MSEL Receptive Language functioning (F(2, 107) = 4.087, p = .019, ηp2 = .071). A similar pattern emerged, such that toddlers diagnosed later (i.e., 25–41M group) earned significantly lower scores than those in the 12–18M group (p = .025), with the difference between receptive language functioning in the 19–24M and 25–41M groups also approaching significance (p = .050). Of note, a main effect of diagnostic age bracket on MSEL Expressive Language scores was not significant (F(2, 106) = 1.759, p = .177, ηp2 = .032). All toddlers in our sample were delayed in their understanding of and attention to oral language, as well as their ability to express themselves using sounds and words (Table 2).
As observed on direct testing with the MSEL, caregiver report on the VABS-II yielded a significant main effect of diagnostic age bracket on VABS-II Motor performance, with a medium effect size (F(2, 108) = 4.646, p = .012, η2 = .079). Motor skills were lowest for the children diagnosed with ASD later in development (i.e., 25–41M group) compared to those diagnosed early (i.e., 12–18M; p = .017) and in our middle group (i.e., 19–24M; p = .031), who did not differ from each other (p = .634).
As observed for MSEL Expressive Language, results did not indicate a significant main effect of diagnostic age bracket on caregiver-reported VABS-II Communication scores (F(2, 108) = 1.541, p = .219, η2 = .028).
Two additional domains of adaptive functioning were investigated using caregiver report on the VABS-II. As shown in Table 2, results revealed a significant main effect of age group on a child’s Daily Living skills (F(2, 108) = 4.518, p = .013, η2 = .077). Toddlers in the 25–41M group had lower daily living abilities than those in both the 12–18M (p = .054) and 19–24M (p = .014) groups; both younger groups had similar daily living skills, based on caregiver report (p = .990). A significant main effect of diagnostic age bracket was also observed for VABS-II Socialization domain scores, with a large effect size (F(2, 108) = 13.737, p < .001, η2 = .203). The 25–41M group had lower adaptive social abilities than both the 12–18M group (p < .001) and the 19–24M group (p = .002), and the 19–24M group further had lower caregiver-reported social skills than the 12–18M group (p = .014).
ASD Symptomatology
As shown in Table 2, diagnostic age bracket was associated with a trend-level difference in total ASD severity on the ADOS-2 (F(2, 109) = 2.706, p = .071, η2 = .047), with this difference driven largely by age-related differences in restricted and repetitive behaviors (F(2, 109) = 4.867, p = .009, η2 = .082), rather than by social communication symptoms (F(2, 109) = .628, p = .536, η2 = .011). Specifically, 25–41M participants exhibited a higher severity of restricted and repetitive behaviors on the ADOS-2 compared to those in both the 12–18M (p = .017) and 19–24M (p = .022) groups; these younger groups did not significantly differ from each other (p = .697).
Results also indicated a significant main effect of diagnostic age bracket on ASD severity as ascertained through both caregiver report and direct clinical observation on the CARS2, with a medium effect size (F(2, 106) = 5.156, p = .007, ηp2 = .089). Similar to patterns observed on the ADOS-2, MSEL, and VABS-II, post-hoc analyses showed that children diagnosed with ASD between the ages of 25 and 41 months exhibited more severe symptoms of ASD than toddlers diagnosed between 12 and 18 months of age (p = .021) or between 19 and 24 months of age (p = .012), who did not differ from each other (p = .849).
Discussion
The current study aimed to explore development and ASD symptoms in young toddlers diagnosed with ASD early (i.e., 12–18 months), in the middle of (i.e., 19–24 months), and later (i.e., 25–41 months) in the early developmental period. Of note, our sample included children drawn from a general population-based screening study rather than an infant sibling sample, unlike previous literature exploring symptom onset patterns in early versus later diagnosed children, and all toddlers in our study were initially screened for ASD prior to age 21 months (i.e., the upper age range of our 18-month screening time point). Furthermore, all three of our groups were diagnosed with ASD earlier than the current national median age at diagnosis (i.e., 3 to 4 years; CDC, 2018), so even our later diagnosed group was still relatively young.
Overall, results revealed greater impairment associated with later diagnosis, such that children diagnosed with ASD between 25 and 41 months showed more significant delays and ASD symptoms than toddlers detected earlier in development. Specifically, three patterns were observed. First, the younger two groups did not differ from each other but were less impaired than the oldest group on visual problem solving, motor functioning, daily living skills, restricted and repetitive behaviors, and overall autism symptom severity. The second pattern was linear, with the youngest group least impaired, followed by the middle group, and with the oldest group most impaired on fine motor skills, receptive language, and socialization and play skills. Finally, all three groups were impaired to a similar extent in certain core areas associated with ASD, particularly expressive language skills and social communication. No significant group differences were found for child sex or family demographic factors. Findings have important implications for ASD screening and treatment practices, especially as children identified earlier in development, when ASD symptoms are less pronounced, may be more amenable to EIBI (MacDonald et al., 2014).
As expected, all children in our sample exhibited delays in development, with only a few exceptions, consistent with their diagnosed neurodevelopmental disorder. Such delays were most apparent in terms of communication and socialization skills, as all toddlers exhibited impaired receptive and expressive language abilities and moderate to severe autism symptoms, regardless of diagnostic age bracket (Table 2). Moreover, our results replicate those of Landa and colleagues (2007) in terms of specific domains of developmental delay in toddlers with ASD, while also confirming the pattern of “progressive divergence from typical development” observed in their sample (Landa et al., 2013, p. 436). That is, our 25–41M group was overall the most delayed and most symptomatic, suggesting progressively greater divergence from a normative developmental trajectory over time.
Contrary to hypothesized results, toddlers diagnosed with ASD prior to age 18 months were not more impaired than those diagnosed with ASD later. Instead, as noted above, we documented a pattern of greater impairment associated with a later diagnosis of ASD, with children in the 25–41M group most severely affected on a majority of the measured areas of development, followed by toddlers in the 19–24M and 12–18M groups. Notably, the children diagnosed prior to age 25 months tended to appear similar in terms of the extent of their delays, though those in the 12–18M group were consistently most mildly affected.
Of interest, a main effect of diagnostic age bracket was particularly significant and large for fine motor skills, such that toddlers diagnosed later demonstrated impaired fine motor functioning, whereas those diagnosed between 19 and 24 months (i.e., the middle group) showed low average fine motor skills, and those children diagnosed before 19 months (i.e., early) exhibited average fine motor abilities. While not considered diagnostic of ASD, fine motor delays have often been linked to later risk for ASD and other developmental disorders (Bhat, Galloway, & Landa, 2012; Bhat, Landa, & Galloway, 2011). In fact, fine motor abnormalities are considered to be neurological ‘soft signs’: clinical impairments associated with a range of neurobehavioral problems, which are possibly related to underlying brain abnormalities (Iannetti, Mastrangelo, & Di Netta, 2005). Thus, the fact that our 25–41M participants demonstrated more severe fine motor delays in addition to their significant cognitive and social delays may suggest that disruptions to normal neural development become more apparent in those children over time as their developmental trajectory shifted further away from a typical course.
Most toddlers in the current study, irrespective of diagnostic age bracket, showed clearly defined and moderately severe ASD symptoms, both on direct testing with the ADOS-2 and by caregiver and clinician report on the CARS2. Children diagnosed between the ages of 25 and 41 months exhibited more severe restricted and repetitive behaviors than the two younger groups. Restricted, repetitive patterns of behavior and interests tend to manifest later in the preschool years (Stone et al., 1999), but when they do present in younger children (i.e., before 18 months) with ASD, they are often in a milder form compared to social communication deficits (Miller, Burke, Robins, & Fein, 2019). The greater severity of ASD symptoms in later diagnosed children may also be a product of a possibly greater underlying neurological abnormality, or it might instead be reflective of the increased amount of time during which symptoms developed (but were not intervened upon) relative to participants diagnosed before two years.
There are several reasons why children diagnosed with ASD later in development (i.e., 25 to 41 months of age) may demonstrate more significant developmental delays and ASD severity. First, toddlers in the 25–41M group might have had a regressive subtype of ASD, such that they lost previously acquired social communication skills, or a later presenting form of ASD, such that they developed atypical behaviors following initial ASD-specific screening. In support of this hypothesis, slightly more than one third of participants in the 25–41M group screened negative at their initial screening time point at or before 18 months of age but then screened positive and were identified with ASD at 24- or 36-month screening (Figure 1). A regressive phenotype is a reported phenomenon in ASD (Barton et al., 2012; Kim et al., 2018; Lord et al., 2012; Luyster et al., 2005), and recent data suggest that regressive onset patterns in children with ASD occur much more frequently than previously recognized, with a majority of high risk children who go on to display ASD showing declines in early social behaviors such as eye contact (Ozonoff & Iosif, 2019). However, the current study did not include a reliable measure of regression. It is equally possible that children diagnosed later exhibited “mixed features” or an otherwise atypical developmental course (e.g., plateauing, stagnation), consistent with prior literature on ASD symptom onset patterns (Ozonoff et al., 2010). Initial negative screening results at or before 18 months could also be due to inaccurate caregiver reporting.
Furthermore, while groups did not differ on any demographic or family factors, contrary to our hypothesis, other more difficult-to-measure family attributes (e.g., caregiver understanding of typical development, caregiver denial of child delays, complex psychosocial stressors) may impact diagnostic timing. Despite the push toward earlier identification of and intervention for ASD, many caregivers and pediatric providers are reluctant to pursue developmental concerns in early childhood, particularly before the age of 18 months, instead employing a ‘wait and see’ approach or erroneously assuming that the child will outgrow mild delays (Dai et al., 2019). This was indeed the case for some children in the current study, as in some cases an evaluation was declined until a second positive screening at 18, 24, or 36 months of age. As a result, fewer toddlers are detected very early, either because of this apparent denial or unwillingness to acknowledge delays, or because their ASD symptoms are more subtle prior to age 25 months.
Additionally, as children get older and symptoms become more pronounced (e.g., in our 25–41M group), developmental delays are much more difficult to ignore or interpret as benign or transient. Opportunities to observe toddlers, particularly those without older siblings, compared to their typically developing peers are much more frequent as children enter the third year of life, as many children transition to daycare or pre-school programs around that age. Thus, the gap between typically developing and developmentally delayed toddlers, particularly in terms of their communication and socialization skills, becomes more evident towards this later point in early development, and caregivers may be more willing to pursue evaluation and intervention (Dai et al., 2019). Unfortunately, in clinical and research practice, it is challenging to ascertain the true reasons for families’ reluctance to attend developmental evaluations for their children beyond a lack of parental concerns and scheduling constraints, both of which certainly impacted the timely provision of evaluation services for some participants in this study.
It is important to acknowledge the potential impact of biases in measurement on findings. For example, measures include fewer items to assess development in the very youngest children because they are capable of performing fewer skills at those ages. Unless they are substantially delayed, younger children, including those in our 12–18M and 19–24M age groups, could achieve higher scores on standardized developmental measures. Conversely, participants in our 25–41M group may have scored lower on standardized developmental measures because, based on their ages, they would be expected to perform more advanced skills. These measurement differences may be more inflated on the MSEL due to the use of developmental quotient scores, which are particularly impacted by age. That is, as children get older, the score denominator, chronological age, continuously gets larger, yet the numerator, mental age (i.e., AE score), increases at a much slower pace in lower functioning children. However, scores that are less affected by age, namely VABS-II standard scores, ADOS-2 comparison scores, and CARS2 total scores, demonstrated a pattern of delays similar to the MSEL in our sample.
Finally, differences in screening times and outcomes were certainly associated with child diagnostic age bracket in our sample. As shown in Figure 1, all the participants in the 12–18M group were assigned to begin screening before 18 months, with most randomized to initial screening at age 12 months. This allowed for an average age at initial positive screen of 13 months, with age at diagnosis averaging around 15 months for those children (Table 1). While some children in the 19–24M group were screened at 12- or 15-month time points, the majority screened positive at age 18 months and were subsequently evaluated. Indeed, as shown in Table 1, the average age at initial positive screen for the 19–24M group was 18.5 months, with evaluations occurring on average at 21 months. Nearly 90 percent of children in the 25–41M group were randomized to have initial screening at 18 months, as opposed to either 12 or 15 months. However, an interesting pattern emerged in this group, such that over one third of those children screened negative at their initial screening and positive at a subsequent rescreening, close to half screened positive at their initial screening but evaluations were deferred by caregivers until a later positive screening at 24 or 36 months, and approximately one fifth were evaluated slightly later due to an unavoidable scheduling time lag (Figure 1). This contributed to an average age at initial positive screen of 22 months, with an average age at evaluation of 30 months (Table 1). Our results suggest that earlier screening can facilitate earlier age at diagnosis and can possibly detect milder delays. That said, it is not possible to know what children diagnosed between 25 and 41 months of age would have looked like clinically had they been screened and evaluated earlier in their development. Given how many participants in the 25–41M group were identified later due to initial caregiver refusals of evaluations, it appears that strongly encouraging early evaluation at the first sign of concerns could be a useful point of intervention for pediatricians and other clinicians.
Limitations and Future Directions
There are some limitations to this study that should be noted. Most significantly, given the design of the broader study, which aimed to determine an ideal screening schedule for ASD, not all children were screened before 18 months. Therefore, it is possible that at least some of our toddlers diagnosed later, particularly those in the 25–41M group, might have been detected earlier if they were first screened at either 12 or 15 months as were children in our 12–18M group. Nonetheless, participating pediatric practices were randomized to screening schedules, thereby reducing bias in terms of who was screened earlier. Additionally, as in the real world, not all caregivers agreed to pursue a developmental evaluation as soon as this was offered, and sometimes there was an unavoidable time lag until evaluation. Differences in “external factors,” including screening practices and referral and evaluation pathways, likely present real barriers to timely diagnosis for children, and our project attempted to reduce the impact of these external factors to the extent possible by facilitating early screening and providing opportunities for free developmental and diagnostic evaluations to families. While these efforts may not generalize to non-research settings, our results do suggest that encouraging evaluation as soon as ASD suspicion arises, whether from positive screening or caregiver or physician concerns, may be beneficial in detecting ASD earlier and in a milder form.
Furthermore, while we were able to assess a variety of demographic and family factors (i.e., “child factors”) that may have been associated with diagnostic timing, we were not able to fully explore potential reasons for more severely affected children being detected later in development. Of course, it is possible that those children were not manifesting symptoms until a later age. That said, exploration of alternative methods of garnering information regarding parental perceptions of ASD diagnostic procedures, including early screening and evaluation, as well as factors influencing interpretation of child delays (e.g., comparison to an older sibling or typically developing peer in daycare) may be indicated, to possibly include qualitative research methods.
Though our sample size was adequate and comparable to other studies of young children with ASD, our early diagnosis group in particular was relatively small (n = 20). While the size of our three groups is generally reflective of population-based patterns of ASD diagnosis, with most cases being detected following 18-month screening, some still being identified after an additional screening at 24 months of age, and relatively few being detected prior to an 18-month screening (as most pediatric practices do not routinely screen for ASD before age 18 months), we recognize the need for comparable data from other studies in order to best understand patterns of symptom onset in ASD.
Finally, we aimed to extend previous work based on infant sibling samples by utilizing a general population-based screening sample, as infant siblings of ASD probands might be distinct from low-risk children (i.e., those without genetic risk for ASD). We were quite successful in establishing a fairly diverse sample, contributing to increased generalizability of our findings, and we had remarkably complete demographic information on most participants. For example, we had maternal race/ethnicity and education data for 98 to 100 percent of our sample, likely due to the fact that we attempted to collect such information at multiple time points (i.e., both at screening and evaluation). We did not collect paternal variables or income data at multiple time points, but we still obtained data from at least 80 percent of our sample. Analysis of missingness suggested no systematic group differences between reporters of demographic information and non-reporters. In the future, it will be important to make concerted efforts to obtain demographic information from participants at multiple time points, as this appears to be a beneficial strategy, and to consistently work to improve diversity and generalizability within our research samples.
Conclusions
The current study extends the limited literature exploring patterns of ASD symptom onset in early versus later diagnosed children in two significant ways: (1) by drawing our sample from a general population-based screening sample, and (2) by comparing children diagnosed before 18 months of age to both those diagnosed between 19 and 24 months and those diagnosed between 25 and 41 months. This approach allowed for a more comprehensive exploration of differences in developmental and social functioning based on diagnostic timing.
The findings reported above, as well as potential reasons for more significant impairment in later diagnosed children, point to the importance of rescreening for ASD at 24 months of age in order to maximize the likelihood of identifying more children and initiating intervention services (Dai et al., 2019). However, our results also suggest that toddlers diagnosed with ASD very early in development, optimally before the age of 19 months but certainly before age two years, show less significant delays and less severe ASD symptoms. It is important to highlight that the parent study from which our participants were drawn employed a comprehensive universal ASD screening program. If such a universal screening program did not exist, and ASD was detected only via caregiver or physician concerns, results might be quite different. Based on our findings, universal screening could be especially impactful and effective in identifying toddlers with milder ASD and more subtle developmental delays, whom might have a better prognosis with earlier initiation of EIBI. Therefore, efforts to increase routine screening before 18 months and to encourage pediatric practices to follow the AAP’s current ASD screening recommendations, in addition to emphasizing the advantages of early evaluation for ASD, may increase the number of children identified early, when intervention is particularly successful (Landa et al., 2007; Landa et al., 2013; MacDonald et al., 2014).
Acknowledgements:
We would like to thank the children and families who participated in this study, as well as the participating pediatric practices who assisted by offering this screening study to their patients. We are also particularly grateful to the research teams at the University of Connecticut, Drexel University, and Georgia State University for their help with recruitment and data collection and management. Data used in the preparation of this manuscript were submitted to the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier(s): DOI: 10.15154/1519057.
Funding: This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD039961).
Footnotes
Declaration of Conflicting Interests: Diana Robins and Deborah Fein are owners of M-CHAT, LLC, which receives royalties from companies that incorporate the M-CHAT-R/F into commercial products or distribute products containing the M-CHAT-R/F. Data reported in this manuscript did not incur any royalties. Lauren Miller and Yael Dai declare that they have no conflicts of interest.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Anderson DK, Liang JW, & Lord C (2014). Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. Journal of Child Psychology and Psychiatry, 55(5), 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT, Watson LR, Crais E, Turner-Brown L, & Reznick S (2013). First year inventory (FYI): Version 3.1 Chapel Hill, NC: University of North Carolina at Chapel Hill. [Google Scholar]
- Barton ML, Dumont-Mathieu T, & Fein D (2012). Screening young children for autism spectrum disorders in primary practice. Journal of Autism and Developmental Disorders, 42(6), 1165–1174. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, & Landa RJ (2012). Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development, 35(4), 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, & Galloway JC (2011). Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy Journal, 9, 1116–1129. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2018). Prevalence of autism spectrum disorder among children aged 8 years – Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. April 27th, Morbidity and Mortality Weekly Report Surveillance Summaries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai YG, Miller LE, Ramsey RK, Robins DL, Fein DA, & Dumont-Mathieu T (2019). Incremental utility of 24-month autism spectrum disorder screening after negative 18-month screening. Journal of Autism and Developmental Disorders. Advance online publication. doi: 10.1007/s10803-019-03959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duby JC, Lipkin PH, Macias MM, Wegner LM, Duncan P, Hagan JF, et al. (2006). Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance and screening. Pediatrics, 118(1), 405–420. [DOI] [PubMed] [Google Scholar]
- Esler AN, Bal VH, Guthrie W, Wetherby A, Weismer SE, & Lord C (2015). The autism diagnostic observation schedule, toddler module: standardized severity scores. Journal of Autism and Developmental Disorders, 45(9), 2704–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VB, Hyman SL, Johnson CP, Bryant J, Byers B, Kallen R, … Yeargin-Allsopp M (2007). Identifying children with autism early? Pediatrics, 119(1), 152–153. [DOI] [PubMed] [Google Scholar]
- Harris SL, & Handleman JS (2000). Age and IQ at intake as predictors of placement for young children with autism: A four-to six-year follow-up. Journal of Autism and Developmental Disorders, 30(2), 137–142. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ … the IBIS Network. (2017). Early brain development in infants at high risk for autism spectrum disorder. Nature, 542, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy LE, Brooks B, Dumont-Mathieu T, Barton ML, Fein D, Chen C-M, & Robins DL (2014). Standardized screening facilitates timely diagnosis of autism spectrum disorders in a diverse sample of low-risk toddlers. Journal of Developmental and Behavioral Pediatrics, 35(2), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AJ, Miller LE, & Fein DA (2017). Autism spectrum disorders and low mental age: Diagnostic stability and developmental outcomes in early childhood. Journal of Autism and Developmental Disorders, 47, 3967–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, & Lord C (2014). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44(10), 2400–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti P, Mastrangelo M, & Di Netta S (2005). Neurological “soft signs” in children and adolescents. Journal of Pediatric Neurology, 3, 123–125. [Google Scholar]
- IBM Corp. (2019). IBM SPSS Statistics for Macintosh, Version 26.0 Armonk, NY: Author. [Google Scholar]
- Johnson CP, & Myers SM (2007). American academy of pediatrics, council on children with disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics, 120(5), 1183–1215. [DOI] [PubMed] [Google Scholar]
- Kim SH, Bal VH, Benrey N, Choi YB, Guthrie W, Colombi C, & Lord C (2018). Variability in autism symptom trajectories using repeated observations from 14 to 36 months of age. Journal of the American Academy of Child and Adolescent Psychiatry, 57(11), 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, & Garrett-Mayer E (2007). Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry, 64(7), 853–864. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, & Faherty A (2013). Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child Development, 84(2), 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Luyster RJ, Gotham K, & Guthrie W (2012). Autism diagnostic observation schedule, second edition (ADOS-2) (Part II): Toddler module [Manual]. Torrance, CA: Western Psychological Services. [Google Scholar]
- Lord C, Luyster R, Guthrie W, & Pickles A (2012). Patterns of developmental trajectories in toddlers with autism spectrum disorder. Journal of Consulting and Clinical Psychology, 80, 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism diagnostic observation schedule, second edition (ADOS-2) (Part I): Modules 1–4 [Manual]. Torrance, CA: Western Psychological Services. [Google Scholar]
- Luyster R, Richler J, Risi S, Hsu W, Dawson G, Bernier R, … Lord C (2005). Early regression in social communication in autism spectrum disorders: A CPEA study. Developmental Neuropsychology, 27(3), 311–336. [DOI] [PubMed] [Google Scholar]
- MacDonald R, Parry-Cruwys D, Dupere S, & Ahearn W (2014). Assessing progress and outcome of early intensive behavioral intervention for toddlers with autism. Research in Developmental Disabilities, 35(12), 3632–3644. [DOI] [PubMed] [Google Scholar]
- Mandell DS, Novak MM, & Zubritsky CD (2005). Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics, 116(6), 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pedraza FL, & Carter AS (2009). Autism spectrum disorders in young children. Child and Adolescent Psychiatric Clinics of North America, 18(3), 645–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Burke JD, Robins DL, & Fein DA (2019). Diagnosing autism spectrum disorder in children with low mental age. Journal of Autism and Developmental Disorders, 49(3), 1080–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning. Circle Pines, MN: American Guidance Services. [Google Scholar]
- Osterling JA, Dawson G, & Munson JA (2002). Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology, 14, 239–251. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Gangi D, Hanzel EP, Hill A, Hill MM, Miller M, … Iosif A (2018). Onset patterns in autism: Variation across informants and timing. Autism Research, 11, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, & Iosif AM (2019). Changing conceptualizations of regression: What prospective studies reveal about the onset of autism spectrum disorder. Neuroscience & Biobehavioral Reviews, 100, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A-M, Baguio F, Cook IC, Hill MM, Hutman T, … Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry, 49(3), 256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, … Iosif A-M (2015). Diagnostic stability in young children at risk for autism spectrum disorder: A baby siblings research consortium study. Journal of Child Psychology and Psychiatry, 56(9), 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Gazestani VH, Bacon E, Barnes CC, Cha D, Nalabolu S, … Courchesne E (2019). Evaluation of the diagnostic stability of the early autism spectrum disorder phenotype in the general population starting at 12 months. JAMA Pediatrics. Advance online publication. doi: 10.1001/jamapediatrics.2019.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel J, Summers J, Lorv B, Szatmari P, Zwaigenbaum L, Georgiades S, & Duku E (2013). Pilot randomized controlled trial of a functional behavior skills training program for young children with autism spectrum disorder who have significant early learning skill impairments and their families. Research in Autism Spectrum Disorders, 7(11), 1418–1432. [Google Scholar]
- Robins DL, Casagrande K, Barton M, Chen CMA, Dumont-Mathieu T, & Fein D (2014). Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics, 133(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, & Vismara LA (2008). Evidence-based comprehensive treatments for early autism. Journal of Clinical Child & Adolescent Psychology, 37(1), 8–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Van Bourgondien ME, Wellman GJ, & Love SR (2010). Childhood autism rating scale, second edition (CARS2) [Manual]. Torrance, CA: Western Psychological Services. [Google Scholar]
- Sparrow SS, Cicchetti DV, & Balla DA (2005). Vineland adaptive behavior scales (2nd ed.). Circle Pines, MN: American Guidance Services. [Google Scholar]
- Stone WL, Lee EB, Ashford L, Brissie J, Hepburn SL, Coonrod EE, & Weiss BH (1999). Can autism be diagnosed accurately in children under 3 years? Journal of Child Psychology and Psychiatry, 40(2), 219–226. [PubMed] [Google Scholar]
- Wetherby A, & Prizant B (2001). Communication and symbolic behavior scales developmental profile: Preliminary normed edition. Baltimore, MD: Paul H. Brookes Publishing Co. [Google Scholar]
- World Health Organization. (2004). International statistical classification of diseases and related health problems (10th rev., 2nd ed.). Geneva, Switzerland: Author. [Google Scholar]


