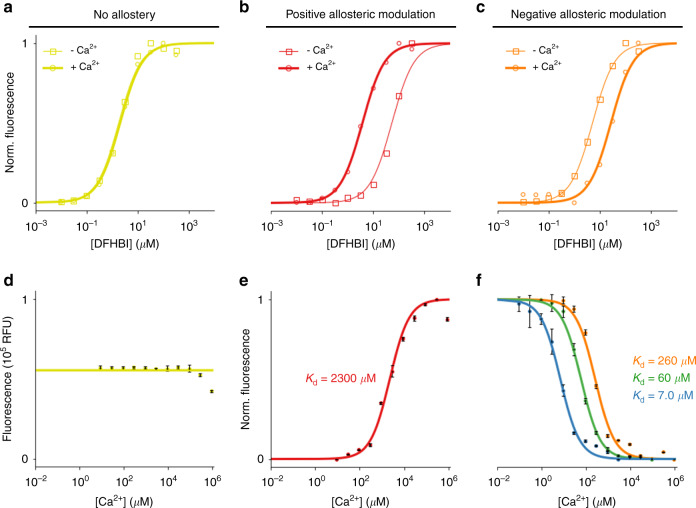
Fig. 4. In vitro characterization of Ca2+-responsive mFAPs.
a–c DFHBI titration in the absence of Ca2+ (squares) and presence of Ca2+ (circles). a For mFAP2b, Ca2+ does not affect DFHBI binding. b For EF1p2_mFAP2b, binding of Ca2+ and DFHBI exhibit positive allostery. c For EF1n_mFAP2b, binding of Ca2+ and DFHBI exhibit negative allostery. a–c Normalized fluorescence intensities (n = 1) are fit to a sigmoid function using non-linear least squares fitting (lines). d–f Ca2+ titrations with excess DFHBI concentration compared to protein concentration. d Unnormalized mean fluorescence intensities of mFAP2b demonstrating a lack of Ca2+-responsiveness. e Normalized mean fluorescence intensities of EF1p2_mFAP2b (with one EF-hand motif, Kd = 2300 µM) demonstrating positive allostery between DFHBI and Ca2+ binding. f Ca2+-responsiveness is dependent on the number of EF-hand motifs inserted into loop7, as exemplified by the normalized mean fluorescence intensities of EF1n_mFAP2b (with one EF-hand motif, Kd = 260 µM), EF2n_mFAP2b (with two EF-hand motifs, Kd = 60 µM), and EF4n_mFAP2b (with four EF-hand motifs, Kd = 7.0 µM), demonstrating negative allostery between DFHBI and Ca2+ binding. d–f Error bars represent the s.d. of the mean of three technical replicates. The means (n = 3) are fit to a d constant function, or e sigmoid or f inverse sigmoid function with Hill coefficients of 1, using non-linear least squares fitting (lines) to obtain Kd values (Supplementary Table 2).