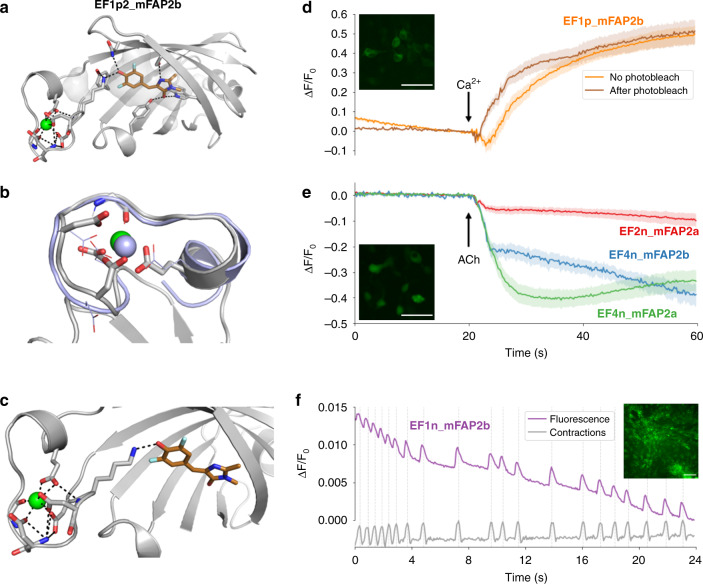
Fig. 5. Structural and in cell characterization of Ca2+-responsive mFAPs.
a–c X-ray co-crystal structure of EF1p2_mFAP2b bound to DFHBI and Ca2+ at 2.1 Å resolution. a Structure of monomer with Ca2+ (green sphere) and DFHBI (copper sticks) bound, with protein side-chains (gray sticks) forming first- and second-shell hydrogen bonds with Ca2+ and DFHBI (black dotted lines), and vacuum electrostatic contact potential around DFHBI (transparent gray surface). b Aligned is the nuclear magnetic resonance (NMR) solution structure from PDB accession code 1NKF (violet cartoon and lines) with La3+ ion bound (violet sphere) having the closest Cα-Cα root mean square deviation (2.19 Å) to the structure of the grafted EF-hand motif (gray sticks and cartoon) with bound Ca2+ ion (green sphere). c Co-crystal structure of EF-hand motif residues (gray sticks) reveals that residue K101 from the EF-hand motif directly hydrogen bonds (black dotted lines) with DFHBI, suggesting an allosteric coupling mechanism between Ca2+ (green sphere) and DFHBI (copper sticks) binding. d Fluorescence imaging of Ca2+ titrations (beginning at arrow) of live HEK293 cells expressing extracellular EF1p_mFAP2b, demonstrating positive allostery in cyto. Average fluorescence fold-change (lines) and s.e.m. (shading) is shown for regions of interest (ROIs) surrounding single cells without photobleaching (orange; n = 15) or after a photobleaching challenge (dark orange; n = 15) from three technical replicates. e Fluorescence imaging of acetylcholine (ACh) stimulations (added at arrow) of live HEK293 cells expressing cytosolic either EF2n_mFAP2a (red), EF4n_mFAP2b (blue), or EF4n_mFAP2a (green), demonstrating negative allostery in cyto. Average fluorescence fold-change (lines) and s.e.m. (shading) are shown for ROIs surrounding single cells expressing EF2n_mFAP2a (n = 15), EF4n_mFAP2b (n = 10), or EF4n_mFAP2a (n = 15) from three technical replicates (Table 2). f Fluorescence imaging of live human induced pluripotent stem cell-derived cardiomyocytes expressing sarcoplasmic reticulum-targeted EF1n_mFAP2b (violet) labeled with DFHBI at approximately (Supplementary Table 2) showing whole field of view normalized fluorescence fold-change, demonstrating negative allostery in cyto. The normalized average of three ROI traces in the fluorescence channel (gray) indicate peak cardiac contraction frames (dotted lines). d–f Representative whole field of view pseudocolored maximum intensity z-axis projections. Scale bars represent d, e 50 and f 100 microns. Source data is available for Fig. 5d–f.