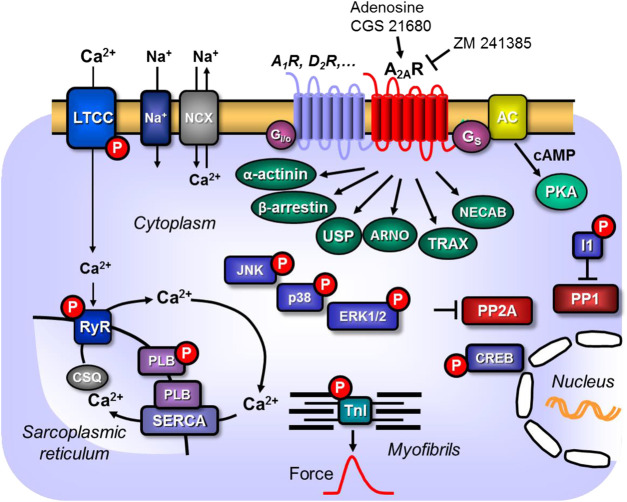
Figure 1.
Scheme: Putative mechanism(s) of signal transduction of cardiac A2A-adenosine receptors (A2a-ARs). A2a-ARs via stimulatory G-proteins (Gs) can activate adenylyl cyclase (AC) which would enhance the 3′-5′cyclic adenosine-phosphate (cAMP)-levels in compartments of the cardiomyocyte and activate cAMP-dependent protein kinases (PKA) which would increase the phosphorylation state and thereby the activity of various regulatory proteins in the cell. Moreover, phosphorylation state and thus the activity of ERK1/2, JNK, p38 and CREB could be enhanced by pathways via arrestins. PKA-stimulated phosphorylation might also increase the current through the L-type Ca2+ channel (LTCC) and/or release of Ca2+ from the sarcoplasmic reticulum (SR) via the cardiac ryanodine receptor (RYR2); both processes would increase force of contraction by increasing the Ca2+ acting on myofilaments. In diastole, Ca2+ is pumped via the SR-Ca2+ ATPase (SERCA) from the cytosol into the SR. Activity of SERCA is increased by phosphorylation of phospholamban (PLB). The latter effect might also follow from inhibition of PP2A (a serine/threonine phosphatase: PP) activity by MAP kinases and subsequent increased phosphorylation state and thus activation of I-1 (a specific inhibitory protein of PP1) which will lead to decreased activity of PP1. Reduced activity of PP2A (and/or PP1) can increase phosphorylation of additional proteins and might thus increase the Ca2+ -sensitivity of myofilaments by dephosphorylation of the myosin light chains in the myofilaments which would increase force of contraction. Thus, A2A-ARs might increase the Ca2+ -sensitivity of myofilaments. In addition, cardiac A2A-ARs might act via the non-canonical pathway of β-arrestin, via α-actinin, via the Arf nucleotide site opener/cytohesin-2, ubiquitin-specific processing protease, translin-associated protein-X and neuronal calcium-binding protein 2.