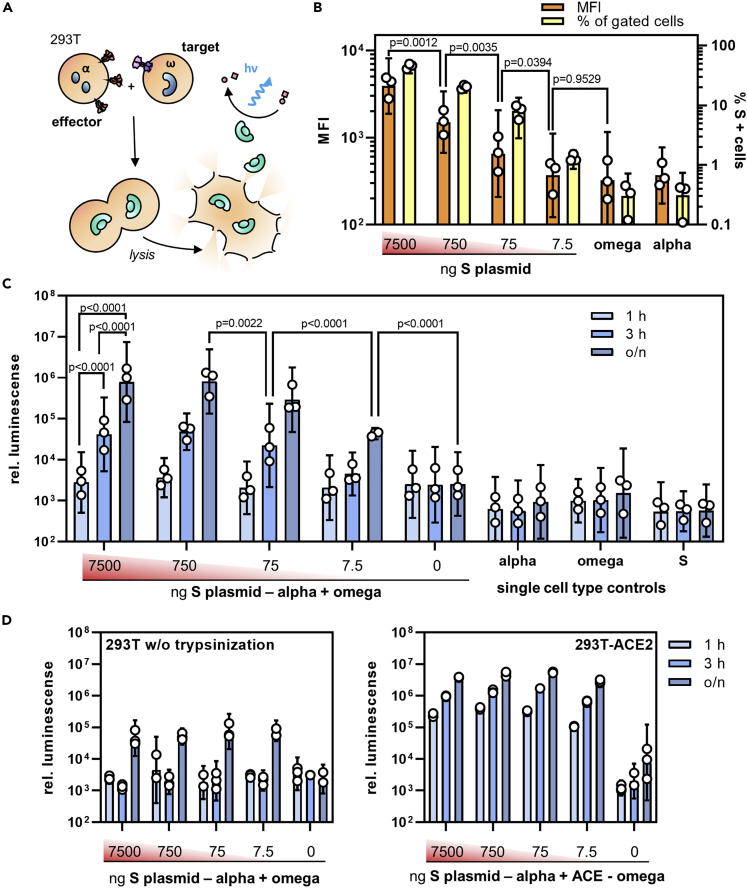
Figure 2.
Quantifying S protein-mediated cell-cell fusion
(A) Experimental setup for the quantification of cell-cell fusion. Active β-galactosidase is formed when effector cells expressing S protein and the α-fragment fuse with target cells expressing ACE2 and the ω-fragment.
(B–D) Effector cells transfected with different amounts of S-protein expression plasmid (ranging from 7500 ng to 7.5 ng per T75 flask) were assessed for S protein expression by flow cytometry (B) and then used in the fusion assay (C-D). (B) Mean fluorescence intensity (MFI, orange bars) and the percentage of S-positive cells (yellow bars) were determined in flow cytometry. Bars represent (geometric) means ±95% confidence intervals (CIs), n = 3. p values are from one-way analysis of variance (ANOVA).
(C) Activities of β-galactosidase in cocultures of effector and target cells in absence of ACE2 overexpression. Effector cells were transfected with the indicated amounts of S protein encoding plasmid, detached with trypsin and cultivated for the indicated time periods (o/n: overnight) with target cells which were transfected with the ω-fragment encoding plasmid only. Bars represent geometric means ±95% CIs, n = 3. p values are from two-way ANOVA.
(D) Influence of proteolytic processing and ACE2 overexpression on cell fusion activity. Left panel: Effector and target cells were detached without trypsin using 5 mM EDTA in PBS. Right panel: Target cells were co-transfected with the ω-fragment and ACE2 encoding plasmids. Bars represent geometric means of technical triplicates ±95% CIs.
See also Figure S1.