Figure 3. Validation of C6-sensitive WDR5 interaction partners.
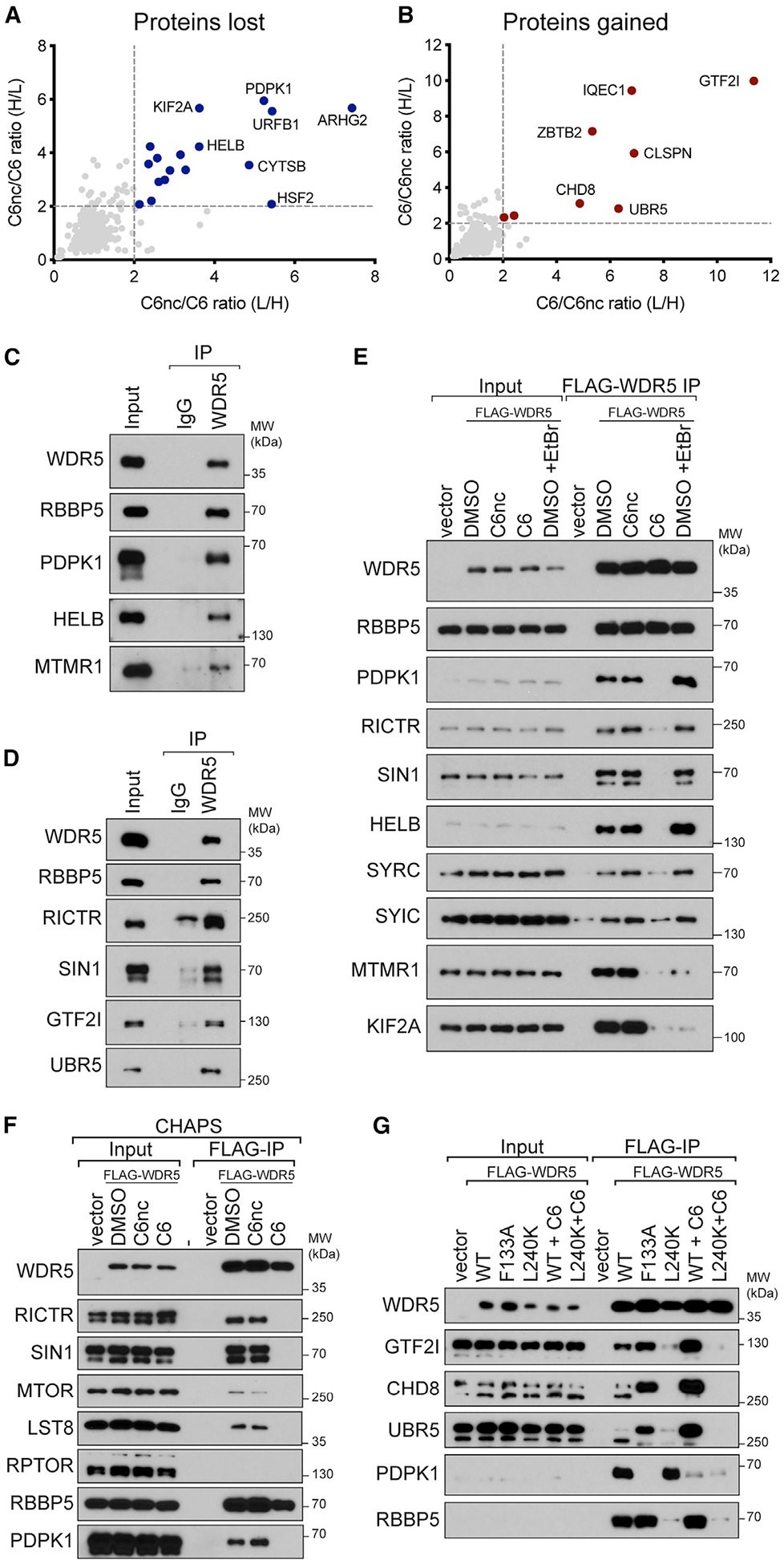
(A) Comparison of C6nc/C6 ratios for the two SILAC replicates. Depleted proteins that met a 2-fold cutoff in both replicates are highlighted in blue.
(B) As in (A) except for enriched proteins (red).
(C) Extracts from HEK293 cells were subject to IP with a polyclonal antibody against WDR5 or an immunoglobulin G (IgG) control. IP samples were probed with antibodies against the indicated endogenous proteins. Inputs are 2% for WDR5 and RBBP5 and 0.3% for others. n = 3 biological replicates.
(D) As in (C) but for different candidate proteins. Inputs are 5% for WDR5, RBBP5, and UBR5 and 0.3% for others. n = 3 biological replicates.
(E) HEK293 cells stably expressing FLAG-tagged WDR5 were treated for 4 h with 30 μM C6 or C6nc prior to lysis and subsequent FLAG IP. For ethidium bromide (EtBr) treatment, 200 μg/mL EtBr was added to the lysate for the duration of the experiment. Candidate WDR5 interaction partners were probed by IB. Inputs are 5% for WDR5 and RBBP5, 0.1% for SYRC and SYIC, and 1% for all others; n = 3 biological replicates.
(F) HEK293 cells stably expressing FLAG-tagged WDR5 were treated for 4 h with 30 mM C6 or C6nc prior to lysis and subsequent FLAG IP in buffer using CHAPS detergent. IP samples were probed with antibodies against the indicated proteins. Inputs are 10% for WDR5 and RBBP5 and 1% for others; n = 3 biological replicates.
(G) HEK293 cells stably expressing FLAG-tagged WDR5 proteins were treated for 4 h with 30 μM C6 (where indicated) prior to lysis and FLAG IP. IP samples were probed with antibodies against the indicated proteins. Inputs are 10% for WDR5 and 1% for others; n = 3 biological replicates.
See also Figure S3.
