Abstract
Background
Atypical antipsychotics as first-line drugs have been used in patients with schizophrenia in China and abroad. However, its safety still needs to be evaluated in a large population, especially in Chinese patients.
Objective
The main objective of this study is to evaluate the safety and related factors of long-term atypical antipsychotic use in patients with schizophrenia in China. The secondary objective includes the long-term efficacy of atypical antipsychotics in these patients, as well as pharmacoeconomic evaluation, population pharmacokinetic studies and pharmacogenomics studies.
Methods
This study has an observational design. The atypical antipsychotics include quetiapine, olanzapine, risperidone, aripiprazole, ziprasidone, paliperidone, amisulpride, perospirone and clozapine. Visits occur at 0, 4, 8, 13, 26, 52, 78, 104, 130 and 156 weeks. The efficacy evaluations include symptoms, social function, recurrence rate and hospitalisation. The safety measures include physical examination, vital signs, abdominal circumference, laboratory tests (such as blood cell analysis, blood biochemical tests and serum prolactin/thyroxine levels), 12-lead ECG, extrapyramidal syndrome assessment, sexual function evaluation, medication and other adverse events. The secondary measures include the Positive and Negative Syndrome Scale, Clinical Global Impression-Severity of Illness Scale, Calgary Depression Scale for Schizophrenia, Personal and Social Performance Scale, relapse rate, drug consolidation, medical-related expenses, income, drug plasma concentration and genetic information.
Results
This is a large sample, non-interventional and long-term prospective clinical study designed to truly reflect the specific details of clinical practice, fully respect patients’ needs, and understand patients’ treatment intentions and actual treatment details.
Conclusions
This research method details the aims, methods, study design, strengths and limitations of the study.
Keywords: psychiatry
Strengths and limitations of this study.
The study is designed as close to clinical practice as possible, this study design try to reduce the limitations of the study itself, without affecting patients’ drug selection and use, and will obtain data and information from ‘actual clinical practice’ to facilitate the discovery of the safety problems of long-term atypical antipsychotic use.
The limitation of this study is that the sample size needs to be as large as possible, so the workload of this study is large. In addition, due to the nature of our study, we have not predefined statistical methods.
Introduction
Schizophrenia is a serious chronic mental illness with a prevalence of approximately 1%.1 Many cases occur in early adulthood, and social functions are severely impaired.2 Antipsychotics are the main drugs for the treatment of schizophrenia. With the advent of atypical antipsychotic drugs, schizophrenia treatment may be more effective and better tolerated than in the past.3 4 At present, the use of atypical antipsychotic drugs has steadily increased and has now become the first-line treatment for schizophrenia.5 However, current clinical data indicate that long-term use of atypical antipsychotics may cause damage to the cardiovascular, metabolic and central nervous systems, among others.6 The Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study and the Clinical Antipsychotic Trials of Intervention Effectiveness compared typical antipsychotics and atypical antipsychotics, but found no difference in the rate of improvement in mental symptoms and quality of life.7 8 However, some studies have found that atypical antipsychotics have advantages in treating negative symptoms, enhancing cognition, reducing extrapyramidal symptoms (EPSs) and improving subjective experience and tolerance.9
In addition, there are many problems in clinical treatment using atypical antipsychotics. Currently, clinicians rely more on their own experiences during drug selection.10 In clinical practice, trial and error strategies are common, which may prolong the treatment cycle and artificially increase the treatment cost.11 Besides, there are significant individual differences in the efficacy and adverse reactions of antipsychotics, presenting different results, while the current treatment model is based on modern pharmacology. Modern pharmacological research objects are groups rather than individuals.12
In view of the aforementioned situation, a large number of studies have been conducted. Among them, there are many reports about the short-term efficacy and safety of atypical antipsychotics, but relatively few on the long-term efficacy and safety, and most are studies done outside China.13 14 Moreover, most of these studies are randomised controlled trials. To obtain more reliable research conclusions with a limited sample size, more inclusion and exclusion criteria are determined to control the interference factors. Such subjects are not representative, and it is difficult to reflect the ‘real-world’ effects of atypical antipsychotics in a large patient population.15 Besides, randomised controlled trials are mostly limited to the analysis of a single factor or several factors and lack the analysis of all factors and their interactions. Therefore, it is difficult to fully understand the factors influencing the long-term prognosis of patients.16
Objective
The main purpose of this study is to comprehensively evaluate the safety of atypical antipsychotic drugs in the treatment of patients with schizophrenia in China and to form an optimised clinical drug treatment plan for clinical practice. Moreover, the second purpose is to evaluate the efficacy of atypical antipsychotics. The efficacy evaluations will include symptoms, social function, recurrence rate and hospitalisation.
Study design
This study is designed as an open, cohort, multicentre, case-only, protocol-modifiable, prospective and observational study representing real-world patients with long follow-up periods (figure 1). Researchers are responsible for maintaining the subjects’ anonymity. Case report forms or other documents can only identify subjects by uppercase alphanumerics and codes. The researchers must keep a record of the participants’ enrolment that indicates the participant’s code, name and home address and keep the documents that show the subject’s identity confidential.
Figure 1.
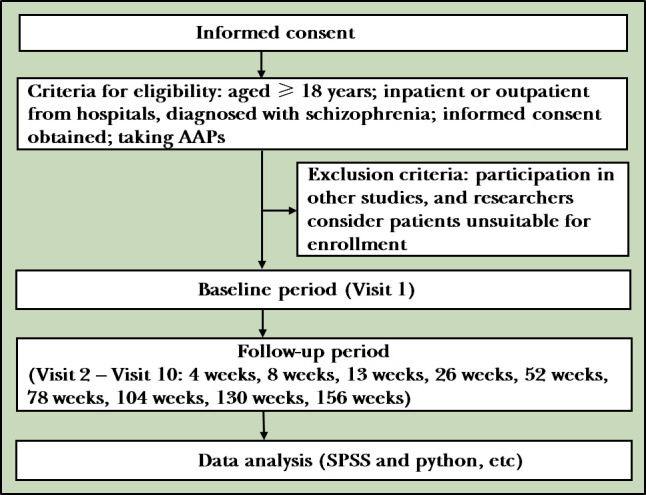
Flowchart of the study. (AAPs: Atypical antipsychotics)
The ethics committee of the Shanghai Mental Health Centre reviewed and approved the protocol. The approval number is 2010–35. This project is registered on the International Clinical Trails Registry Platform (www. clinicaltrials. gov NCT02640911). The brief title on the International Clinical Trails Registry Platform is ‘An Observational Study on Atypical Antipsychotics Long-term Treatment Patients with Schizophrenia’.
Subjects
Subjects should meet the following inclusion criteria: (1) an inpatient or outpatient (man or woman) aged ≥18 years; (2) a diagnosis of schizophrenia by DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition); (3) being able to effectively communicate with the investigator, complete study-related documents, comprehend the key components of the consent form and provide written informed consent to participate in the study prior to any study-specific assessments or procedures, and (4) taking or willing to take atypical antipsychotics (AAPs), including quetiapine, olanzapine, risperidone, aripiprazole, ziprasidone, paliperidone, amisulpride, perospirone and clozapine. Exclusion criteria are as follows: (1) participation in other clinical studies, and (2) other conditions that, by the investigator’s judgement, render patients unsuitable for the clinical study.
Pharmacological treatment
The drugs to be used in this study are atypical antipsychotics, including quetiapine, olanzapine, risperidone, aripiprazole, ziprasidone, paliperidone, amisulpride, perospirone and clozapine. There is no limitation on the actual dosage. The clinician may adjust the drug according to the drug instruction and clinical experience (approved drug instructions for the daily doses are shown in table 1). Because this study is an open, realistic study that does not interfere with the patient’s drug, it is important to record all drugs (including other prescription drugs, over-the-counter drugs and Chinese medicine) during the study period. During drug therapy, adjusting the dosage and changing the drug will be allowed. The researchers will need to keep detailed records of the subjects’ drug use, especially antipsychotics, including overdose, underdose and missed medication. For those whose dose is adjusted and the drug is changed, researchers will need to record the reason, dose and start-stop time. In addition, this study can be combined with any clinically needed drug. The reason, dose and start-stop time of the combined drug should be recorded.
Table 1.
Reference dose
| Medication | Daily dose |
| Quetiapine | 150–750 mg/d |
| Olanzapine | 5–20 mg/day |
| Risperidone (oral solution/injection microspheres) | 2–6 mg/d/25–50 mg/2 weeks |
| Aripiprazole | 10–30 mg/d |
| Ziprasidone | 40–160 mg/d |
| Paliperidone | 3–12 mg/d |
| Amisulpride | 400–800 mg/d |
| Perospirone | 12–48 mg/d |
| Clozapine | 100–600 mg/d |
Clinical and laboratory observation outcomes
The main outcomes will be evaluation of safety, using physical examination, vital signs, abdominal circumference, laboratory tests (such as blood cell analysis, blood biochemical tests, prolactin and thyroxine levels), adverse events, 12-lead ECG, EPS assessment, sexual function evaluation, medication and other subjective feelings. For subjects with EPS, the assessment scale will include the Simpson-Angus Scale(SAS),17 Barnes Akathisia Rating Scale(BARS)18 and Abnormal Involuntary Movement Scale (AIMS).19 The SAS is used to assess extrapyramidal side effects caused by antipsychotic drug treatment and assess gait, arm, head and leg stiffness, and includes a 10-item neurological examination. The internal reliability of the scale is consistent, with a coefficient of 0.79-0.83. The BARS is used to assess the objective and subjective manifestations of akathisia, including 4 neurological examinations, with a score of 0-3 for grade 4 or 1-5 for grade 5. The AIMS is used to assess abnormal movements that may be related to drugs, including 12 neurological examinations. For sexual function evaluation, the Arizona Sexual Experience Scale(ASEX) will be used.20 The ASEX is used to assess the severity of symptoms and adverse reactions of sexual dysfunction caused by drugs. The scale includes 5 items and has good internal reliability consistency. The α-coefficient is 0.89-0.90, and the test-retest reliability is good. the intragroup correlation coefficient is 0.88, the sensitivity is 82% and the specificity is 90%. Regarding the subjective feelings of the subject’s medication, this study will use the Medication Satisfaction Questionnaire(MSQ),21 Drug Attitude Inventory (DAI),22 and the Subjective Well-Being Under Neuroleptics (SWN) to evaluate the patient’s satisfaction and comfort with clinical medication.23 The MSQ is used to assess the satisfaction of patients with schizophrenia taking antipsychotic drugs, including 1 item. The DAI scale is mainly used to assess the treatment compliance of patients with schizophrenia. The reliability correlation coefficient of the scale is 0.61, including 10 items. The SWN is a self-rating scale that is mainly used to asess the perception of quality of life in patients with schizophrenia taking antipsychotic drugs. The internal consistency correlation coefficient is 0.93.
The secondary outcomes will include the efficacy and function assessment scales, relapse rate, drug consolidation, medical-related expenses, income, drug plasma concentration and genetic information. For the efficacy of the drug, the Positive and Negative Syndrome Scale (PANSS) will be used to assess the severity of the symptoms in the subjects24 and Calgary Depression Scale for Schizophrenia (CDSS) to assess the severity of the symptoms associated with depression.25 The PANSS includes 7 items of positive scale, 7 items of negative scale, and 16 items of general psychopathology scale, altogether 30 items. The scale has a high degree og internal reliability and consistency among items. The CDSS includes 9 items, with good internal reliability and consistency. Moreover, the Clinical Global Impression-Severity of Illness Scale (CGI-S) will be used to assess the clinical efficacy of the subject.26 The internal reliability consistency of positive symptoms and total score of the scale is high, and the correlation coefficient within the group is 0.71-0.73. For functional evaluation, the Personal and Social Performance (PSP) scale will be used to assess the social function of patients,27 and the Short Form (SF)-36 health survey28 will be used to investigate the subjects’ quality of life (table 2). The internal reliability consistency of the PSP scale is good, and the α-coefficient is 0.87. The SF-36 scale has good internal reliability consistency, and the α-coefficient is 0.76–0.92.
Table 2.
Scales assessment
| EPS | Self-Rating Anxiety Scale Behavioural Activity Rating Scale Abnormal Involuntary Movement Scale |
| Sexual function evaluation | Arizona Sexual Experience Scale |
| Subjective perception of medication | Medication Satisfaction Questionnaire Drug Attitude Inventory The Subjective Well-Being Under Neuroleptics |
| Curative effect | Positive and Negative Syndrome Scale Calgary Depression Rating Scale Clinical Global Impression |
| Functional evaluation | Personal and Social Performance Scale SF-36 Short Form Health Survey |
EPS, extrapyramidal syndrome; SF-36, Short Form-36.
Research process
The informed consent form must be signed and dated by the subjects and their guardian. Each signed informed consent form should be properly kept and filed by the researcher. Considering the particularity of this study, subjects can voluntarily choose whether to participate in population pharmacokinetic research, pharmacogenomics research or extended research. In this study, some research centres may try to use multimedia informed consent. The content of the multimedia informed consent form should be consistent with the written informed consent form, which also needs to be reviewed and approved by the ethics committee.
In this study, after each subject and their family members sign the informed consent, the researchers will collect demographic data and evaluate the patient’s medical history, treatment history, family history, medical costs and social support in detail. Additionally, researchers will collect data on the subjects’ smoking and drinking status, the use of antipsychotics, combined diseases and symptoms, combined medications and physical examination results (including vital signs and abdominal circumference), complete laboratory test results (including routine blood test, liver and kidney function, electrolytes and blood lipids) and electrocardiographic examinations. If the patient is a woman of childbearing age, a urine pregnancy test may be performed if the researchers deem it necessary.
After completing the baseline assessment, a second visit with the patient will be booked (after 4 weeks). During the second to eighth visit (4, 8, 13, 26, 52, 78, 104, 130 and 156 weeks after the baseline), researchers will recognise similar situations and perform the same scale assessment. At the last visit (the 10th visit), the study completion form will be requested, and the patient will be asked if they are willing to participate in an extended long-term follow-up study. If the patient fails to complete 10 visits as planned, the last visit before exiting the study will be conducted according to the content of the 10th visit (table 3).
Table 3.
Research process sheet
| Study duration (weeks) | 0 | 4±1 | 8±1 | 13±1 | 26±1 | 52±2 | 78±2 | 104±2 | 130±2 | 156±2 |
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Informed consent | × | |||||||||
| Collect basic medical history | ||||||||||
| Diagnosed according to DSM-IV diagnostic criteria | × | |||||||||
| Check the group/exclusion criteria | × | |||||||||
| Collect demographics* | × | |||||||||
| Medical history, treatment history, family history | × | |||||||||
| Social support | × | × | × | × | × | × | × | × | × | × |
| Smoking and drinking | × | × | × | × | × | × | × | × | × | × |
| Antipsychotic use | × | × | × | × | × | × | × | × | × | × |
| MECT and psychotherapy | × | × | × | × | × | × | × | × | × | × |
| Concomitant disease and symptoms | × | × | × | × | × | × | × | × | × | × |
| Drug combination | × | × | × | × | × | × | × | × | × | × |
| Safety evaluation | ||||||||||
| Physical examination† | × | |||||||||
| Vital sign examination‡, abdominal circumference | × | x | x | × | × | × | × | × | × | × |
| Routine blood test | × | x | x | × | × | × | × | × | × | × |
| Blood biochemistry§ | × | x | x | × | × | × | × | × | × | × |
| Other¶ | × | x | x | × | × | × | × | × | × | × |
| Pregnancy test (if necessary) | × | x | x | × | × | × | × | × | × | × |
| 12-lead ECG | × | x | x | × | × | × | × | × | × | × |
| Record adverse events | × | x | x | × | × | × | × | × | × | × |
| EPS (SAS/BARS/AIMS) | × | x | x | × | × | × | × | × | × | × |
| Sexual function evaluation (ASEX) | × | x | x | × | × | × | × | × | × | × |
| Subjective perception of medication (MSQ/DAI/SWN) | × | x | x | × | × | × | × | × | × | × |
| Therapeutic evaluation | ||||||||||
| PANSS, CDSS | × | x | x | × | × | × | × | × | × | × |
| CGI-S | × | x | x | × | × | × | × | × | × | × |
| Functional assessment (PSP/SF-36) | × | x | x | × | × | × | × | × | × | × |
| Relapse/readmission | × | x | x | × | × | × | × | × | × | × |
| Other works | ||||||||||
| End sheet†† | × | |||||||||
| Ask whether to enter an extended long-term follow-up study‡‡ | × |
* Demographic data: age, gender, ethnicity, height, weight, occupation, marriage and economic income.
† Physical examination: skin, lymph node, facial features, head and neck (including thyroid), heart, lung, abdomen, limbs, external genitalia, motor system and nervous system.
‡ Vital signs check: temperature, pulse, breathing and blood pressure.
§ Blood biochemistry: liver function (alanine aminotransferase (ALT), aspartate aminotransferase (AST)), kidney function (urea nitrogen (BUN), inosine (Cr)), fasting blood glucose and glycated haemoglobin.
¶ Other: prolactin and thyroxine.
†† Ending records should be completed when the study is terminated early. The follow-up time window should be ±1 week for less than 52 weeks and ±2 weeks for more than 52 weeks.
‡‡ Including subjects who prematurely discontinued the study. Among them, 4 weeks and 8 weeks of follow-up are suitable for patients with acute exacerbation or fluctuating conditions.
This symbol “x”signifies completion of the visit.
AIMS, Abnormal Involuntary Movement Scale; ASEX, Arizona Sexual Experience Scale; BARS, Barnes Akathisia Rating Scale; CDSS, Calgary Depression Scale for Schizophrenia; DAI, Drug Attitude Inventory; MSQ, Medication Satisfaction Questionnaire; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance Scale; SAS, Simpson-Angus Scale; SF-36, Short Form-36; SWN, Subjective Well-Being Under Neuroleptics Scale.
During the study, it is the patient’s responsibility to report any changes in physical or psychological conditions that occur. All information collected in this study will be comprehensively evaluated by trained investigators and then input into a uniform electronic data capture system. Additionally, it is necessary to provide the subjects with the researchers’ contact information, so that subjects can contact the researchers at any time. The researchers should ask for the subjects’ contact information to contact them in time. Furthermore, the researchers should remind the subjects of the visit time before each expected visit and urge the subjects to complete the laboratory test.
Adverse events and management
Adverse events occur after a subject receives a drug and are not necessarily related to the drug, including any new events or events that worsen in severity and frequency compared with the baseline condition, including abnormal results of diagnostic methods (eg, laboratory and physical examinations).29 The adverse events that occur in this study will be evaluated according to symptoms, signs and laboratory tests. The evaluation criteria include adverse events that coincide with the time of medication, and related to known adverse events of the drug, and that cannot be explained by other causes, and disappear after discontinuation or that recur after readministration.
Adverse events during this study must be recorded on a case report form. Adverse events need to be recorded in medical terms and the diagnosis should be given rather than listing the symptoms and signs (eg, cough, runny nose, sneezing and sore throat should be reported as an upper respiratory tract infection). The researchers should take measures to deal with the adverse events as needed, and the measures should be recorded both in the original file and the case report form.
Researchers need to determine whether abnormal laboratory results are clinically relevant and related to research medications. If unexplainable abnormal laboratory values are noted, repeated inspections or follow-up must be performed until the experimental values are within the normal range or they are reasonably adequately explained and recorded on the case report.
Severe adverse events refer to events that require hospitalisation, prolonged hospital stay, disability, affect work capacity, endanger life or cause death and cause congenital malformations during clinical research.29 When a serious adverse event occurs, the researcher must complete a serious adverse event report form and report it to the drug regulatory department, health administration department and ethics committee within 24 hours. The researchers must sign and date the report, and the clinician should respond according to the clinical symptoms.
Data management
In this study, an electronic data management system will be used to directly input data and the data managers will construct the electronic case report form (eCRF) based on the study plan. According to the different identities of researchers, applicants, inspectors and auditors, separate accounts will be created, and different permissions will be granted to access the eCRF.
Only researchers in each centre can see the contents of the centre and have the right to modify the data. Applicants will be limited to viewing all cases, while inspectors and auditors can read the case situation of each centre, but do not have permission to modify the data; however, they can insert comments or questions. The clinical researcher or data entry clerk designated by the researcher will enter the data from the research medical record into the eCRF in a timely and accurate manner; the eCRF is not a raw record, and its content is derived from the medical record and research visit manual. The inspectors will conduct the inspection through the eCRF, and questions can be raised online at any time when issues are found. The researchers will provide the answers online and modify erroneous data, and the auditors can repeat the process if necessary. For questionable data, researchers should respond immediately in the eCRF system. After each subject completes the trial and the response is audited by the inspector, the data administrators lock the data. During the test, as required, the locked data can be exported in real time for midterm analysis. After all test data of all subjects are locked, the data administrator will export data to the designated database, and the final statistical analysis is performed by statisticians.
Statistical methods
Statistical analysis will be performed using SPSS (V.24.0, IBM, Armonk, New York, USA) software and python. Descriptive statistics will be used to indicate the mean, SD, maximum value, minimum value, median, confidence interval, frequency (composition ratio) and so on. For measurement data, t-test and rank sum test will be used. Counting data will use χ² test and Fisher’s exact test. Ridit analysis and Cochran-Mantel-Haenszel statistics will be used for ranked data. For the main efficacy indicators, per-protocol analysis and intention-to-treat analysis will be performed simultaneously.30 For confounding factors before the treatment, as a covariate, the least squares mean of the analysis of covariance (ANCOVA) and its 95% confidence limit or logistic regression will be used to determine the difference in efficacy between groups and eliminate the influence of these factors on the efficacy. The relationship between the total amount of different drugs and the efficacy will be analysed by the scatter correlation method, and the efficacy relationship will be determined by scatter view. Logistic or multiple linear regression models will be used to analyse drug interactions, and ANCOVA or logistic regression of drug efficacy will be performed. The two-sided test will be used as the general statistical test, and p<0.05 will be considered statistically significant.
Discussion
Real-world research evidence is more conducive to guiding clinical practice. Although randomised controlled studies are highly effective, the number of cases is limited, the follow-up period is short and patient selection may lack typicality. In view of clear inclusion or exclusion criteria, the included population may not be able to provide information on the effectiveness of antipsychotic treatment in the real world. This study is a real-world observational study designed to truly reflect the specific details of clinical practice, fully respect patients’ needs and understand patients’ treatment intentions and actual treatment details.
Summary
This article has described the background, purpose, design and research flow of the study and its proposed method of evaluating the efficacy of atypical antipsychotics in the clinical treatment of patients with schizophrenia. The protocol represents an observational study that does not affect patients’ drug selection and use. It provides large-scale safety clinical research data of atypical antipsychotics in Chinese patients with schizophrenia after long-term use. It will evaluate the efficacy and safety of atypical antipsychotics in the treatment of patients with schizophrenia in China based on the patient’s laboratory indicators and clinical symptoms, analyse the influencing factors that affect the long-term outcome of drug therapy, and quantitatively evaluate the weights to form an optimised, popularised clinical drug treatment plan for clinical practice to produce results that benefit clinicians, managers, policy makers and patients. This study may provide evidence for long-term treatment strategies in schizophrenia, concerning atypical antipsychotic selection related to relapse and social function. At present, there are articles published on the analysis of the data in this study, and a pharmacodynamic model has been established to quantify the time–efficacy relationship of antipsychotic drugs.31–33
Biography
Dr Wenjuan Yu obtained a master's degree from the Xiangya Medical College of Central South University, China, in 2007, and a doctorate degree from Shanghai Jiao Tong University, China, in 2015. Since 2007, she has been working at Shanghai Mental Health Center. Currently, she is working as an associate chief physician at Shanghai Mental Health Center, Shanghai, China. Her main research interests include the nerves of schizophrenia cognitive psychology and individualised treatment.

Footnotes
Contributors: WY and SH are responsible for the project implementation and the writing of the paper. JH is responsible for the project implementation and ethical review. LZ is responsible for the project implementation and data collection. YS and HL are responsible for the scientific design of the study.
Funding: This study was supported by the Clinical Research Center, Shanghai Jiao Tong University School of Medicine (DLY201620), National Science and Technology Major Project for IND (2018ZX09734-005), Shanghai Clinical Research Center for Mental Health (19MC1911100), Medical Engineering Cross Project of Shanghai Jiao Tong University(YG2017MS42).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All protocols and materials were approved by the Institutional Review Board of Shanghai Mental Health Center.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 2003;60:1187–92. 10.1001/archpsyc.60.12.1187 [DOI] [PubMed] [Google Scholar]
- 2.van Os J, Kapur S. Schizophrenia. Lancet 2009;374:635–45. 10.1016/S0140-6736(09)60995-8 [DOI] [PubMed] [Google Scholar]
- 3.Leucht S, Corves C, Arbter D, et al. . Second-Generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009;373:31–41. 10.1016/S0140-6736(08)61764-X [DOI] [PubMed] [Google Scholar]
- 4.Meltzer HY Update on typical and atypical antipsychotic drugs. Annu Rev Med 2013;64:393–406. 10.1146/annurev-med-050911-161504 [DOI] [PubMed] [Google Scholar]
- 5.Leucht S, Corves C, Arbter D, et al. . Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009;373:31–41. 10.1016/S0140-6736(08)61764-X [DOI] [PubMed] [Google Scholar]
- 6.Komossa K, Rummel-Kluge C, Schwarz S, et al. . Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2011:Cd006626. 10.1002/14651858.CD006626.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones PB, Barnes TRE, Davies L, et al. . Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1). Arch Gen Psychiatry 2006;63:1079–87. 10.1001/archpsyc.63.10.1079 [DOI] [PubMed] [Google Scholar]
- 8.Naber D, Lambert M. The CATIE and CUtLASS studies in schizophrenia: results and implications for clinicians. CNS Drugs 2009;23:649–59. 10.2165/00023210-200923080-00002 [DOI] [PubMed] [Google Scholar]
- 9.Turner MS, Stewart DW. Review of the evidence for the long-term efficacy of atypical antipsychotic agents in the treatment of patients with schizophrenia and related psychoses. J Psychopharmacol 2006;20:20–37. 10.1177/1359786806071243 [DOI] [PubMed] [Google Scholar]
- 10.Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull 2015;114:169–79. 10.1093/bmb/ldv017 [DOI] [PubMed] [Google Scholar]
- 11.Cutler A, Ball S, Stahl SM. Dosing atypical antipsychotics. CNS Spectr 2008;13:3–14. 10.1017/S1092852900003035 [DOI] [PubMed] [Google Scholar]
- 12.Basile VS, Masellis M, Potkin SG, et al. . Pharmacogenomics in schizophrenia: the quest for individualized therapy. Hum Mol Genet 2002;11:2517–30. 10.1093/hmg/11.20.2517 [DOI] [PubMed] [Google Scholar]
- 13.Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. . Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019;394:939–51. 10.1016/S0140-6736(19)31135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishimoto T, Hagi K, Nitta M, et al. . Long-Term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry 2019;18:208–24. 10.1002/wps.20632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche N, Reddel HK, Agusti A, et al. . Integrating real-life studies in the global therapeutic research framework. Lancet Respir Med 2013;1:e29–30. 10.1016/S2213-2600(13)70199-1 [DOI] [PubMed] [Google Scholar]
- 16.Gelman A Benefits and limitations of randomized controlled trials: a commentary on Deaton and Cartwright. Soc Sci Med 2018;210:48–9. 10.1016/j.socscimed.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 17.Calvo-Gómez JM, Sánchez-Pedraza R, Jaramillo-González LE, et al. . [Validating the Simpson-Angus Extrapyramidal Collateral Symptom Evaluation Scale]. Rev Salud Publica 2006;8:74–87. 10.1590/s0124-00642006000100007 [DOI] [PubMed] [Google Scholar]
- 18.Barnes TRE The Barnes Akathisia Rating Scale--revisited. J Psychopharmacol 2003;17:365–70. 10.1177/0269881103174013 [DOI] [PubMed] [Google Scholar]
- 19.Lane RD, Glazer WM, Hansen TE, et al. . Assessment of tardive dyskinesia using the abnormal involuntary movement scale. J Nerv Ment Dis 1985;173:353–7. 10.1097/00005053-198506000-00005 [DOI] [PubMed] [Google Scholar]
- 20.Byerly MJ, Nakonezny PA, Fisher R, et al. . An empirical evaluation of the Arizona sexual experience scale and a simple one-item screening test for assessing antipsychotic-related sexual dysfunction in outpatients with schizophrenia and schizoaffective disorder. Schizophr Res 2006;81:311–6. 10.1016/j.schres.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 21.Vernon MK, Revicki DA, Awad AG, et al. . Psychometric evaluation of the medication satisfaction questionnaire (MSQ) to assess satisfaction with antipsychotic medication among schizophrenia patients. Schizophr Res 2010;118:271–8. 10.1016/j.schres.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 22.Stjernswärd S, Persson K, Nielsen R, et al. . A modified drug attitude inventory used in long-term patients in sheltered housing. Eur Neuropsychopharmacol 2013;23:1296–9. 10.1016/j.euroneuro.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Vothknecht S, Meijer C, Zwinderman A, et al. . Psychometric evaluation of the subjective well-being under neuroleptic treatment scale (SWN) in patients with schizophrenia, their relatives and controls. Psychiatry Res 2013;206:62–7. 10.1016/j.psychres.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 24.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261–76. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Xiao W. Evaluation of depressive symptoms in schizophrenia - Calgary schizophrenia depression scale. Int J Psychiatry. [Google Scholar]
- 26.Padhi A, Fineberg N. Clinical Global Impression Scales[M. Springer Berlin Heidelberg, 2010. [Google Scholar]
- 27.Qiao Y, He S, Su L, et al. . Applicability of the Chinese version of the personal and social performance scale in patients with severe mental disorders. Asia Pac Psychiatry 2017;9:e12271. 10.1111/appy.12271 [DOI] [PubMed] [Google Scholar]
- 28.Li L, Wang H, Shen Y. Development and psychometric tests of a Chinese version of the SF-36 health survey scales. Zhonghua Yu Fang Yi Xue Za Zhi 2002;36:109–13. [PubMed] [Google Scholar]
- 29.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356:1255–9. 10.1016/S0140-6736(00)02799-9 [DOI] [PubMed] [Google Scholar]
- 30.Heritier SR, Gebski VJ, Keech AC. Inclusion of patients in clinical trial analysis: the intention-to-treat principle. Med J Aust 2003;179:438–40. 10.5694/j.1326-5377.2003.tb05627.x [DOI] [PubMed] [Google Scholar]
- 31.Weng J, Zhang Y, Li H, et al. . Study on risk factors of extrapyramidal symptoms induced by antipsychotics and its correlation with symptoms of schizophrenia. Gen Psychiatr 2019;32:e100026. 10.1136/gpsych-2018-100026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Li Y-G, He S, et al. . Quantitative efficacy of three antipsychotic drugs for schizophrenia based on a real-world study in China. Acta Pharmacol Sin 2019;40:1611–20. 10.1038/s41401-019-0285-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He S, Yu WJ, Wang X, et al. . Risk factors of hyperprolactinemia induced by risperidone and olanzapine and their correlations with plasma glucose and lipids. Gen Psychiatr 2020;33:e100206. 10.1136/gpsych-2020-100206 [DOI] [PMC free article] [PubMed] [Google Scholar]


