Figure 3.
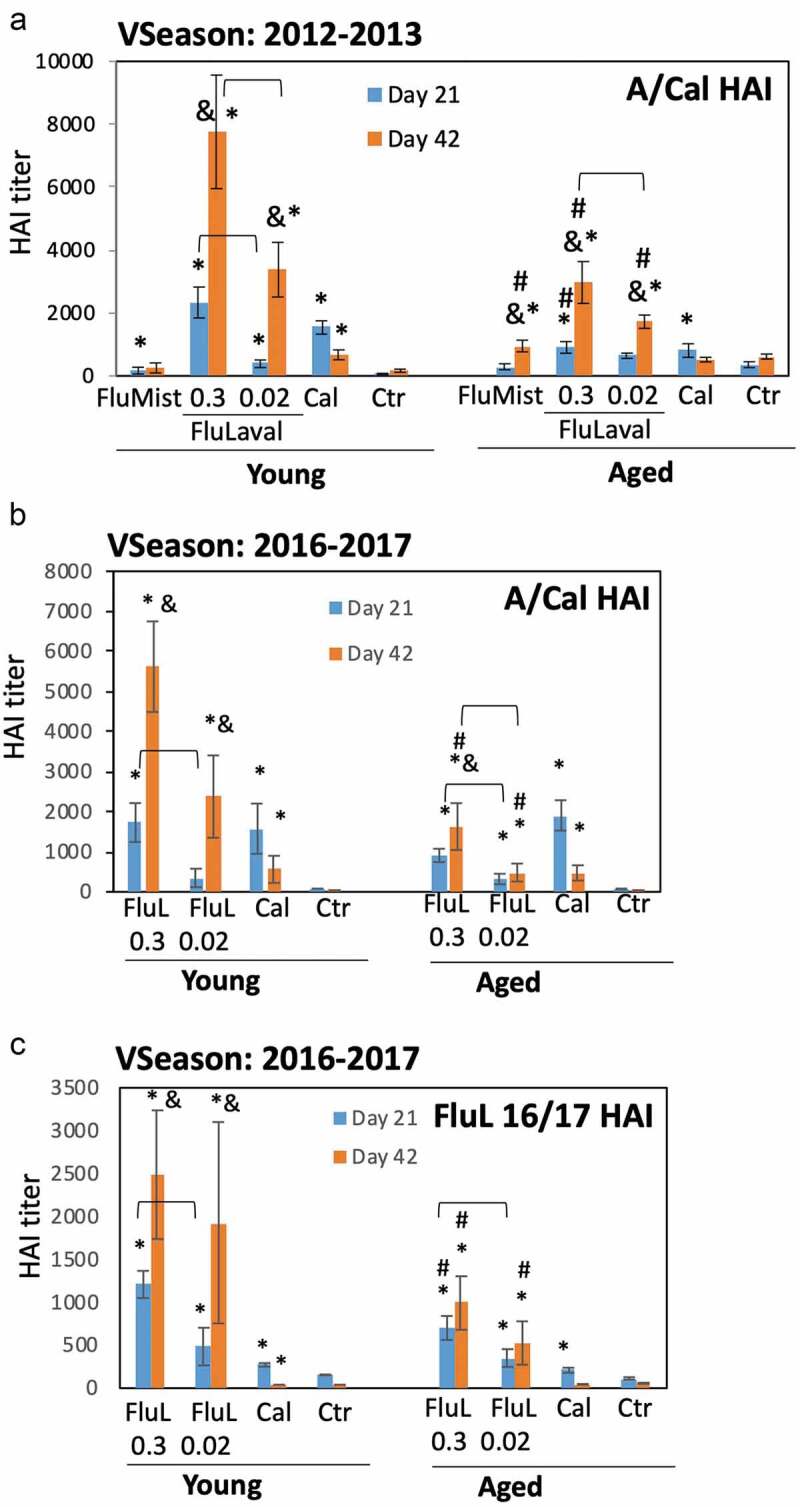
Immunogenicity of seasonal influenza vaccines in the young and aged cotton rats. (a) Immunogenicity of FluLaval 2012–2013 and FluMist 2012–2013 in the young and aged cotton rats. Animals were immunized with the indicated doses of FluLaval 2012–2013 i.m. or with FluMist 2012–2013 i.n. during the corresponding vaccine season and boosted 3 weeks later. Control animals were infected i.n. with influenza A/California (Cal). Serum samples were collected prior to boost and three weeks after the boost for analysis of HAI titers against A/Cal. Results represent GMT±SE for 11–15 animals per group. Negative control animals (Ctr) were unimmunized and uninfected. (b,c) Immunogenicity of FluLaval (FluL) 2016–2017 in the young and aged cotton rats. Animals were immunized as described above but using the FluLaval 2016–2017 formulation during the corresponding vaccine season. Control animals were infected with influenza A/California or left unimmunized and uninfected (Ctr). Blood samples were collected for analysis of serum HAI titers against A/Cal (b) or FluLaval 2016–2017 (c). Results represent GMT±SE for 9–12 animals per group (except for Ctr group, where 5 samples were included). *p< .05 when compared to titers in the same age animals with primary infection, same day; & p< .05 when compared to d21 samples collected after the same dose vaccination for the same age animals; # p< .05 when compared to young animals, same vaccine, dose, and day of collection; ⊓ (connector) p< .05 when compared to the lower vaccine dose in the same age animals, same day of blood collection
