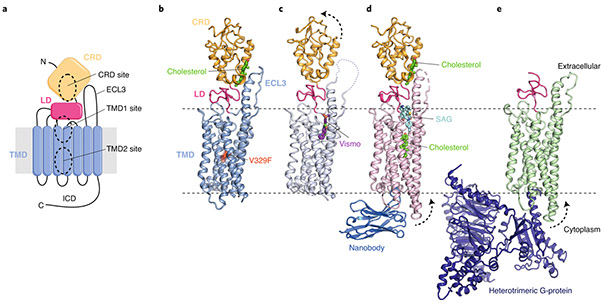
Fig. 2 ∣. Multiple sterol binding sites in SMO.
a, Schematic of SMO showing the extracellular cysteine-rich domain (CRD), linker domain (LD), transmembrane domain (TMD), third extracellular loop (ECL3) and intracellular domain (ICD). The three structurally and functionally characterized small-molecule binding sites (two in the TMD and one in the CRD) are highlighted. N and C refer to N- and C-termini, respectively. b–e, Cartoon representation of the closed SMO–cholesterol complex (b; PDB ID 5L7D), the SMO–Vismodegib (Vismo) complex in an inhibited conformation (c; PDB ID 5L7I), the SMO–cholesterol–nanobody-SAG (SMO agonist) complex in an activated conformation (d; PDB ID 6O3C) and the SMO–G-protein complex (e, PDB ID 6OT0). Dotted arrows indicate movement compared to that of the closed SMO–cholesterol complex (shown in b). Small molecules are labeled and depicted in stick representation. The V329F mutation that locks SMO in a closed conformation is highlighted as a red sphere in b.