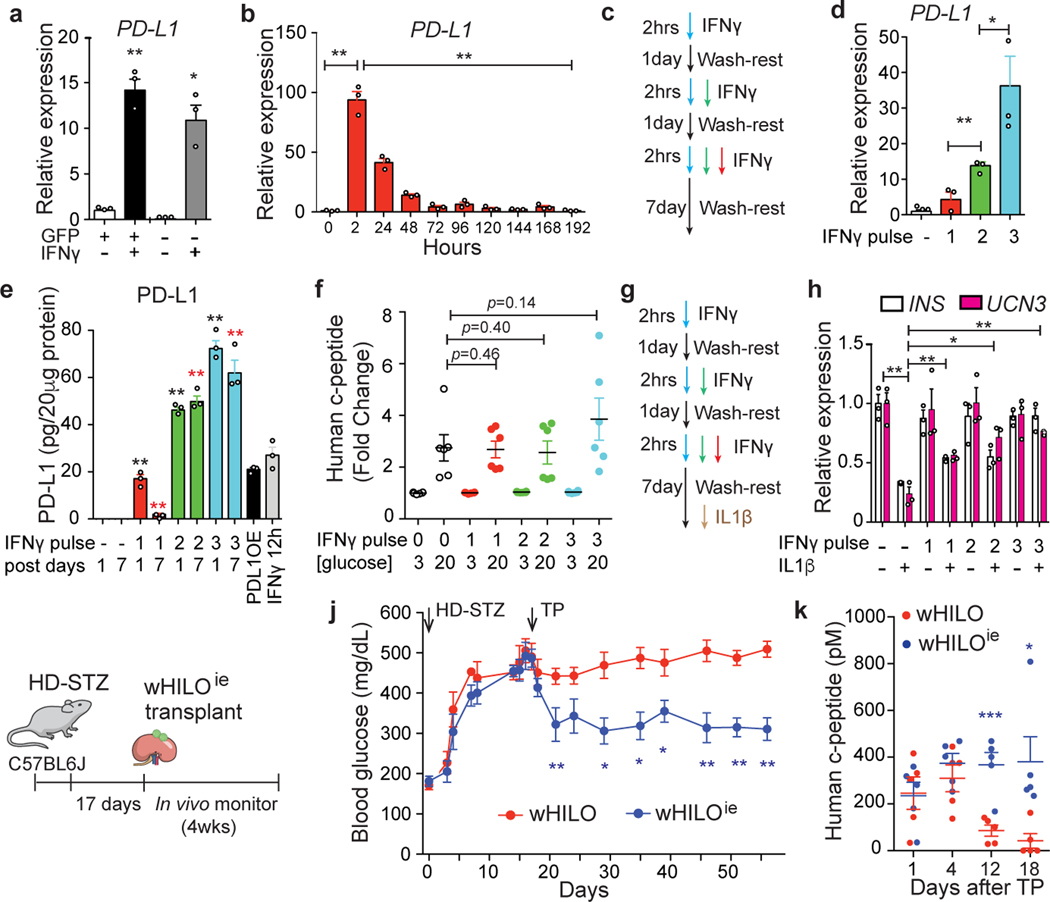
Fig. 4. Enhanced endogenous PD-L1 expression generates immune evasive wHILOs.
a, PD-L1 expression in cells sorted for insulin expression (GFP+ and GFP-, respectively) from wHILOs after IFNγ treatment (10ng/ml, 12 hours). b, Temporal PD-L1 expression in wHILOs after a single IFNγ treatment (10ng/ml, 2 hours). c, Schematic of IFNγ (10ng/ml) pulse treatment. d, PD-L1 expression induced by indicated cycles of IFNγ treatment, 7 days after last treatment. e, PD-L1 protein levels 1 and 7 days after indicated IFNγ (10ng/ml) treatments. PD-L1 overexpressing wHILOs (PDL1OE) and a single 12 h exposure to IFNγ were used as positive control. f, Human c-peptide secretion from IFNγ treated wHILOs in response to 3mM (G3) or 20mM (G20) glucose. g, Schematic of IFNγ treatment in combination with an IL1β challenge (10ng/ml for 24 hours) to induce β cell dedifferentiation. h, INS and UCN3 expression after indicated IFNγ and IL1β treatments of wHILOs. i, Schematic of experimental program. High dose streptozotocin (HD-STZ, 180mg/kg) induced diabetic C57BL6J mice received transplants of 500 wHILOs with or without the IFNγ treatment shown in (c). j, Blood glucose levels in recipient mice after transplantation at day 17 of wHILOs or IFNγ multi pulse-stimulated wHILO (wHILOie) (n=6). k, Serum human c-peptide levels in mice described in (i) (n=5). Error bars represent ± SEM. *p<0.05, **p<0.01, one-tailed, student’s paired t test. n=3 (a,b,d,e,h) and n=6 (e). Data representative (a, b, d, e, f, h) or compiled from (j, k) 3 independent experiments.