Abstract
Studies have shown relationships between white matter abnormalities and cognitive dysfunction in myotonic dystrophy type 1 (DM1), but comprehensive analysis of potential structure-function relationships are lacking. Fifty adult onset DM1 individuals (33 female) and 68 unaffected adults (45 female) completed the WAIS-IV to determine levels and patterns of intellectual functioning. Neuroimages were acquired with a 3T scanner and were processed with BrainsTools. Regional brain volumes (ROIs) were adjusted for inter-scanner variation and intracranial volume. Linear regression models were conducted to assess if group by ROI interaction terms significantly predicted WAIS-IV composite scores. Models were adjusted for age and sex. The DM1 group had lower Perceptual Reasoning Index (PRI), Working Memory Index (WMI), and Processing Speed Index (PSI) scores than the unaffected group (PRI t(113)= −3.28, P = 0.0014; WMI t(114)= −3.49, P = 0.0007; PSI t(114)= −2.98, P = 0.0035). The group by hippocampus interaction term was significant for both PRI and PSI (PRI (t(111)= −2.82, P = 0.0057; PSI (t(112)= −2.87, P = 0.0049)). There was an inverse association between hippocampal volume and both PRI and PSI in the DM1 group (the higher the volume, the lower the IQ scores), but no such association was observed in the unaffected group. Enlarged hippocampal volume may underlie some aspects of cognitive dysfunction in adult-onset DM1, suggesting that increased volume of the hippocampus may be pathological.
Keywords: Cognitive Impairments, Neuroanatomy, WAIS-IV, Hippocampus, Magnetic Resonance Imaging, DM1
Introduction
Myotonic dystrophy type 1 (DM1) is the most common form of muscular dystrophy in adults with a prevalence rate of ~ 1/8,000, and is caused by a CTG trinucleotide repeat expansion in the 3’ untranslated region of the myotonic dystrophy protein kinase gene (DMPK) (D’Angelo & Bresolin, 2006; Meola & Cardani, 2015). DM1 can be categorized into five phenotypes: congenital, infantile-onset, juvenile-onset, adult-onset, or late-onset with distinct clinical features for each phenotype (De Antonio et al., 2016; Gourdon & Meola, 2017; Modoni et al., 2004; van der Plas et al., 2019). Adult onset, or “classic” DM1 has clinical features which may include weakness, myotonia, cataracts, respiratory failure, cardiac defects, and central nervous system (CNS) pathology. With such a variety of pathophysiological symptoms and brain involvement, DM1 has been called a multisystemic, ‘complex network disorder’ (van Dorst et al., 2019).
Although myotonic dystrophy is defined as a muscular disease, altered cognitive functioning is an important feature of DM1 (Meola & Sansone, 2007). Cognitive function varies among the subtypes of DM1 with congenital patients exhibiting major impairment compared with adult-onset patients (Douniol et al., 2012). Nonetheless, individuals with adult-onset DM1 can still experience cognitive difficulties that may be linked with frontal lobe function (Callus et al., 2018; Gallais, Gagnon, Mathieu, & Richer, 2016; Modoni et al., 2004). In a 9-year longitudinal analysis of cognitive function in adults with DM1, Gallais et al. (2016) reported stable verbal IQ and decreased processing speed in complex executive functioning tasks. Some have argued that in patients with relatively small CTG repeat expansions, cognitive decline may be the only observable clinical manifestation of DM1 (Modoni et al., 2004).
Previous studies have found that while adult-onset DM1 patients typically score within broad normal limits on measures of general intelligence and cognitive status, scores tend to be lower than age- and education- matched controls (D’Angelo & Bresolin, 2006; Meola & Sansone, 2007; Rubinsztein, Rubinsztein, McKenna, Goodburn, & Holland, 1997). Additionally, many studies have found significantly poorer cognitive performance in DM1 patients than healthy controls, specifically in measures of visuospatial abilities and perceptual reasoning (Caso et al., 2014; Censori, Danni, Del Pesce, & Provinciali, 1990; Hamilton et al., 2018).
Evaluation of the functional consequences of neuroanatomical abnormalities in DM1 is important for gauging clinical impact (Minnerop, Gliem, & Kornblum, 2018). In their review, Minnerop and colleagues (2018) noted that flexibility of thinking was correlated with GM volume in the secondary visual cortex, delayed recall of verbal memory was correlated with temporal gyri and supramarginal gyrus, and visuospatial impairment was correlated with ventricle enlargement and volume loss in corpus callosum, cingulate isthmus, right occipital, and pericalcarine cortex (Minnerop et al. 2018). Gourdon and Meola (2017) noted that variable cognitive impairments in episodic memory, executive function, and spatial and visuo-constructive abilities are common, even in least-severe form of DM1, and have been associated with widespread atrophy in brain structure. More research is needed to understand how changes in the brain may drive cognitive challenges observed in the DM1 population.
Previously, our group reported on the structural volumetric differences between a large sample of adult-onset DM1 patients and controls, showing significant volume reductions in frontal and parietal gray matter, subcortical structures (putamen, thalamus) and the corpus callosum. Two subcortical regions, the hippocampus and amygdala, were significantly enlarged in the DM1 group relative to controls. (van der Plas et al., 2019). The current study is a follow-up to van der Plas et al. (2019) to evaluate associations between cognitive abilities and volumetric brain structure in adult-onset DM1 patients compared to unaffected adults.
Methods
Participants
Individuals with DM1 were recruited to the University of Iowa via advertisements through the Myotonic Dystrophy Foundation (MDF) and word of mouth. Unaffected participants were primarily recruited from the Iowa City area via advertisements. Some were unaffected spouses of affected participants (N=6) and some control participants had a family history of DM1, but were gene-non-expanded themselves (N=7). Recruitment for baseline assessments took place between September 2014 and September 2018.
Exclusion criteria for all participants were as follows: a learning disability in childhood, a history of serious head injury, or a chronic neurological disorder other than DM1. Unaffected participants were additionally required to be without a history of substance abuse, psychiatric disease, or major medical diseases (i.e. heart disease, sleep disorder, vascular disease, uncontrolled hypertension, cancer, diabetes mellitus, lung disease, and autoimmune conditions). Recruitment was targeted to adult-onset DM1 only, meaning that individuals were included if they denied symptoms before the age of 18 years old.
Participants underwent genetic testing as part of the research study. The majority of patients (n=46) had also undergone genetic testing prior to participation and were aware of their genetic status. A subset of participants was at-risk for DM1 but had not undergone predictive testing (n = 11). At-risk individuals who were determined to have CTG repeat length > 50 were included in the DM1 group (n = 4). The remainder had CTG repeat length in the non-disease associated range and were included in the control group (n = 7). Research staff, clinicians and scientists involved in this study remained blind to the genetic status of at-risk individuals. All data were de-identified and all participants consented to non-disclosure of genetic results obtained as part of the study. Control participants (n = 61) were genotyped for CTG repeat length, to confirm unaffected status. All participants gave written, informed consent prior to enrolling in the protocol in accordance with the Declaration of Helsinki. The study was approved by the University of Iowa Institutional Review Board.
Education
Years of education were obtained using a self-report measure. High school completion was equal to 12 years of education, a bachelor’s degree was equal to 16 years of education, a master’s degree was equivalent to 18 years of education, and 20 years of education was equal to a PhD or higher graduate degree.
Muscle impairment
The extend of muscular impairment in DM1 patients was assessed with the Muscular Impairment Rating Scale (MIRS), which is an ordinal five-point scale ranging from 1 (asymptomatic) to 5 (severe proximal weakness) based on clinical evaluation of muscular involvement (Mathieu, Boivin, Meunier, Gaudreault, & Bégin, 2001).
Cognitive measures
Neuropsychological testing was conducted by research assistants under the supervision of a board-certified clinical neuropsychologist (DJM). Participants completed the Wechsler Adult Intelligence Scale-IV (WAIS-IV) to measure intelligence with four indices that measure Verbal Comprehension (VCI), Working Memory (WMI), Processing Speed (PSI), and Perceptual Reasoning (PRI). VCI measures verbal abstract thinking, word knowledge, and basic factual information to solve novel problems. WMI measures attention, mental math ability and working memory. PSI measures mental speed/efficiency and PRI measures visuoconstructional ability, nonverbal abstract thinking, and complex visuospatial processing (Whitaker, 2013). Composite scores have a normed mean of 100 and standard deviation (SD) of 15. Scores that were at least two SD below or above the normed mean were considered extreme and removed from analysis (N= 2 for WMI and N= 2 for PRI).
Image acquisition
Iowa participants were scanned using either a 3T Siemens Trio TIM (Siemens AG, Munich, Germany; n=52, 12-channel head coil) or a 3T General Electric Discovery MR750w (GE Medical Systems, Chicago, IL; n = 66, 16-channel head and neck coil). Anatomical T1-weighted images via 3T Siemens (coronal MPRAGE, TR = 2300 ms, TE = 2.82 ms, TI = 900 ms, flip angle = 10°, FOV = 282 × 282 × 264 mm, matrix = 256 × 256 × 240) and T2-weighted images were acquired (coronal, TR = 4800 ms, TE = 430 ms, FOV = 256 × 256 × 224 mm, matrix = 256 × 256 × 160). Anatomical T1-weighted images via 3T GE (coronal BRAVO, TR= 8.392 ms, TE= 3.184 ms, TI= 450 ms, flip angle= 12°, FOV = 282 × 282 × 264 mm, matrix = 256 × 256 × 240) and T2-weighted images were acquired (coronal, TR = 3000 ms, TE = 85.925 ms, FOV = 256 × 256 × 224 mm, matrix = 256 × 256 × 160).
Image processing
Bias field inhomogeneity was corrected using the N4 algorithm implemented in Advanced Normalization Tools software (Tustison et al., 2010). Images were processed using the BRAINSAutoWorkup pipeline that incorporates a series of packages and tools under the umbrella of BRAINSTools which optimizes tissue classification through an iterative framework, producing robust results in a multi-site setting (Pierson et al., 2011; Young Kim & Johnson, 2013). BRAINSAutoWorkup utilizes joint label fusion for cortical and subcortical regions based upon the Desikan-Killiany atlas (Desikan et al., 2006; Wang et al., 2013). Scanner effects were harmonized using a ComBat harmonization procedure which estimates prior distributions for each scanner using an empirical Bayesian approach (Fortin et al., 2018; Johnson, Li, & Rabinovic, 2007), as implemented by the ez.combat toolbox in R. We confirmed that scanner did not predict regional volume (see Supplementary table 1) and conducted statistical analyses on the harmonized neuroimaging data.
Statistical Analysis
Volumetric regions of interest (ROIs) were corrected for intracranial volume (ICV) using the power-proportion method (PPM - ROI= α * ICV^β; where β is estimated from non-linear regression models) (Liu et al., 2014). ROIs included in this analysis were regions that were previously found to be different between DM1 patients and unaffected controls (van der Plas et al., 2019; frontal GM, frontal WM, parietal GM, caudate, putamen, nucleus accumbens, thalamus, hippocampus, amygdala, and corpus callosum). Analyses were performed using RStudio version 3.5.1 (Feather Spray).
Differences in cognitive performance between unaffected adults and individuals with DM1 were evaluated using robust linear regression models, with group, age and sex included as predictor variables. Potential impact of muscle impairment was evaluated in the DM1 group by replacing the group variable with a binned “mild” (MIRS ≤ 2) and “severe” (MIRS ≥ 3) muscle impairment variable as a predictor variable. Structure-function associations were evaluated using robust linear regression models with cognitive performance as the dependent variable and the group by ROIppm interaction effect as the predictor variable. Age and sex were included in the models.
Ad-hoc analyses evaluated the impact of CTG length on cognition in the DM1 group via linear regression models to predict cognitive performance while including age and sex in the model as predictor variables. To account for the skewed distribution, we took the square root of CTG repeat length in DM1-affected adults. Multiple comparisons were controlled for by employing the false discovery rate (FDR) and denoted by q-values. FDR < 10% (q < 0.10) was considered statistically significant.
Results
Sample
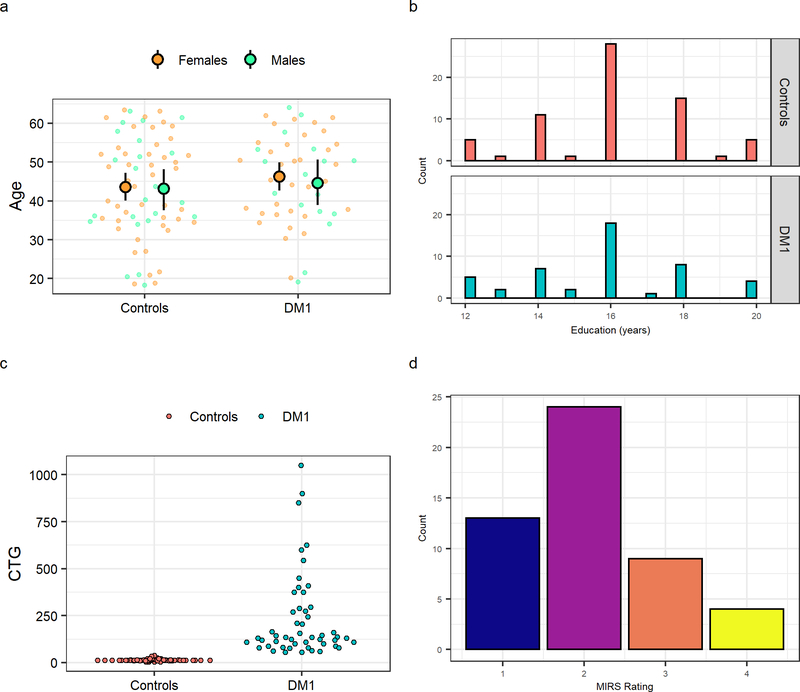
The sample was comprised of 68 unaffected adults (45 female) and 50 DM1-affected adults (33 female) with comparable sex distributions between groups (t(105.5) = 0.02; p = 0.9842). The present sample is an expansion of the Iowa sample described in a previous publication that included cohorts from Iowa and Scotland (van der Plas, 2019). Note that 38 unaffected and 40 DM1-affected adults from the present sample were described previously as part of the Iowa sample. Mean age at evaluation was comparable between groups: unaffected adults were 43.6 years old on average (SD=12.9 years) and DM1 patients 46.7 years old (SD=11.3 years) (Figure 1a). Education levels were also comparable (t(95.113) = 0.72; p = 0.4708) between the control group (M= 16.10, SD =2.07) and DM1-affected participants (M= 15.81, SD =2.20), where 16 years indicated a 4-year college degree (Figure 1b). CTG repeats ranged from 5 to 38 in the control group (M=14, SD=5), and from 55 to 1050 in the DM1 group (M=236, SD=224) (Figure 1c). Distribution of muscle impairment ratings in the DM1 group is shown in Figure 1d. Finally, 37 DM1 patients (74%) had no or mild muscle impairment and 13 (24%) had moderate to severe muscle impairment.
Figure 1:
Sample characteristics. Panel a shows the age distributions of unaffected individuals and DM1- affected adults, separated by sex. Large circles represent the means with 95% confidence limits and the small circles represent individual observations Sex distribution was not different between groups (X2 (1, N = 118) = 6.63 e−31, p = 1). Panel b illustrates the distribution of years of education distribution for unaffected individuals (top panel) and DM1-affected adults (lower panel). Education distribution (binned as less than 4-year college degree [< 16 years] and completed 4-year college degree or higher [≥ 16 years]) was not different between groups (X2 (1, N = 114) = 0.38, p = 0.54). Panel c depicts CTG trinucleotide repeat expansion for each unaffected adult and DM1-affected individual in the sample. Panel d shows Muscle Impairment Rating Scale (MIRS) distribution of DM1-affected adults. Scores of MIRS ≤ 2 indicate none to mild impairment and scores of MIRS ≥ 3 indicate moderate to severe muscle impairment.
Cognitive performance group differences
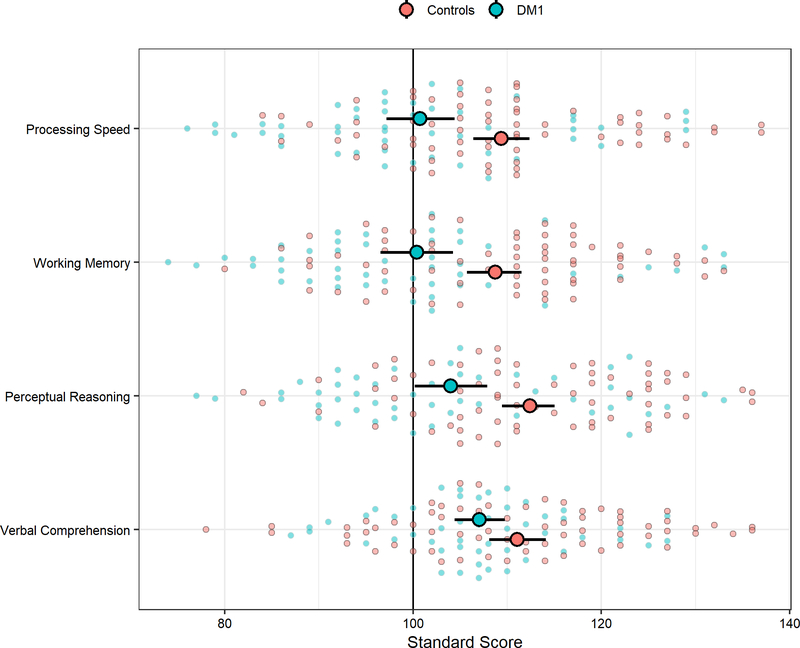
DM1 patients scored significantly lower than unaffected adults on measures of Working Memory (t(116)= −3.49, q = 0.0027), Perceptual Reasoning (t(113)= −3.28, q = 0.0026), and Processing Speed (t(116)= −2.98, q = 0.0046; see Figure 2). Scores on Verbal Comprehension were more similar between the groups (t(114)= −1.69, q = 0.0937). In a subgroup analysis of cognitive performance in the DM1 sample, severe muscle impairment was associated with lower cognitive performance than mild muscle impairment on muscle-heavy tasks measuring Perceptual Reasoning (t(48)= −3.22, q = 0.0082) and Processing Speed (t(48)= −3.01, q = 0.0082), but not for non-muscle-heavy tasks measuring Verbal Comprehension (t(48)= −1.62, q = 0.1496) and Working Memory (t(48)= −1.15, q = 0.2566).
Figure 2:
Cognitive performance group differences between unaffected adults and DM1-affected adults. The vertical, black line marks the normed mean of 100. The larger circles represent the means and 95% confidence limits. Small, transparent circles represent individual observations. Scores > 2.5 SDs above the mean were removed for analysis.
Associations between brain structure and function
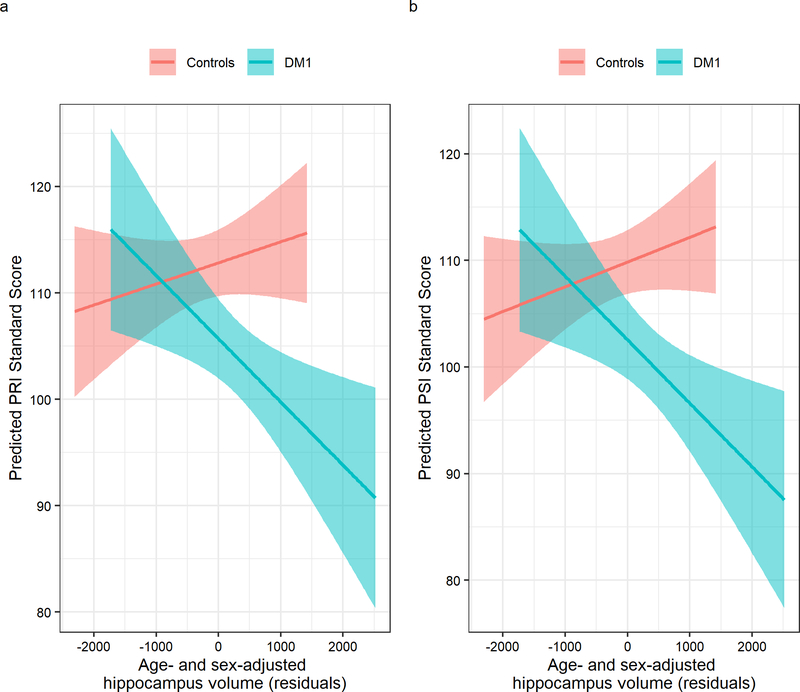
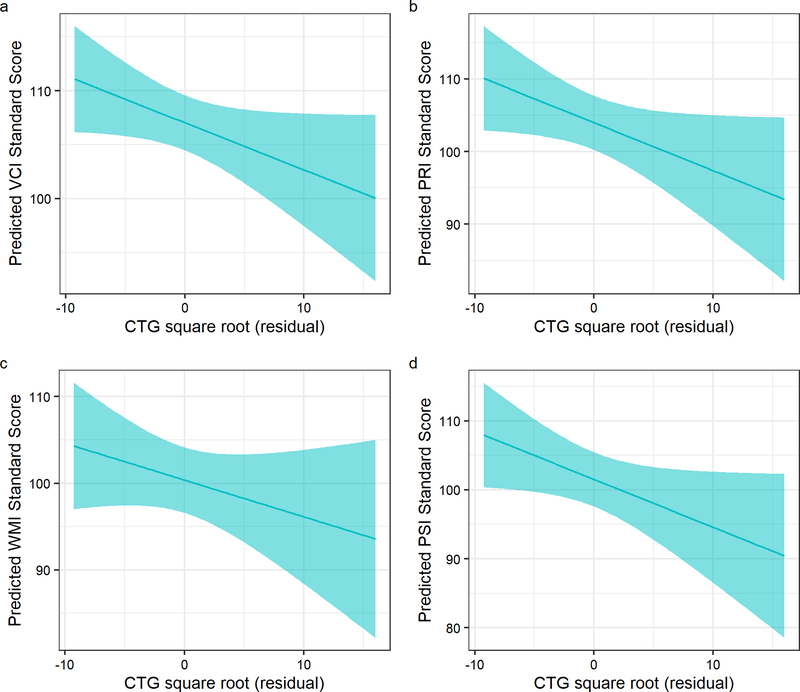
The group by hippocampus interaction term was significant for PRI (t(111)= −2.82, q = 0.0623) and for PSI (t(112)= −2.87, q = 0.0537), indicating that larger hippocampal volume corresponded with lower PRI and PSI scores in the DM1 group, but not in unaffected adults (Figure 3a–b). Statistical summaries for interaction effects of the cognitive domains and ROIs can be found in Table 1. Similar trends were observed for the caudate and corpus callosum, with larger ROI volume being associated with lower cognitive performance scores in the DM1 group: the group by caudate interaction predicted VCI and WMI, and the group by corpus callosum interaction predicted PRI. However, these effects did not remain significant after correcting for multiple comparisons (Table 1). We explored the impact of CTG repeat on cognitive function in DM1-affected adults and observed trends suggesting that higher CTG repeats were associated with worse cognitive performance (Table 2; Figure 4a–d).
Figure 3:
Associations between cognitive functions and hippocampus volume. Panel a illustrates the group by hippocampus (x-axis) interaction effect predicting Perceptual Reasoning (y-axis). Hippocampus volumes are expressed as age- and sex-adjusted residuals. Panel b shows the interaction between group and hippocampus volume predicting Processing Speed.
Table 1.
Cognitive performance predicted by ROI
| ROI | VCI |
PRI |
WMI |
PSI |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta† | t (df) | P | beta† | t (df) | P | beta† | t (df) | P | beta† | t (df) | P | |
| ICV | −0.3 | −0.22 (112) | 0.823 | 1.72 | 1.16 (111) | 0.25 | 0.5 | 0.33 (112) | 0.744 | −0.44 | −0.29 (112) | 0.773 |
| Frontal Lobe GM | 1.54 | 0.19 (112) | 0.851 | 7.15 | 0.87 (111) | 0.385 | −11.38 | −1.28 (112) | 0.204 | −4.37 | −0.49 (112) | 0.623 |
| Frontal Lobe WM | 12.43 | 1.6 (112) | 0.112 | 10.56 | 1.17 (111) | 0.234 | −1.97 | −0.22 (112) | 0.827 | 1.58 | 0.18 (112) | 0.86 |
| Parietal Lobe GM | 0.93 | 0.06 (112) | 0.949 | 9.97 | 0.67 (111) | 0.504 | −2.86 | −0.18 (112) | 0.86 | 9.57 | 0.63 (112) | 0.53 |
| Caudate | −691.21 | −2.33 (112) | 0.022 * | −285.26 | −0.84 (111) | 0.4 | −776.27 | −2.41 (112) | 0.017 * | −176.32 | −0.52 (112) | 0.604 |
| Putamen | −87.96 | −0.37 (112) | 0.715 | 61.09 | 0.23 (111) | 0.815 | −125.84 | −0.5 (112) | 0.618 | −266.31 | −1.06 (112) | 0.29 |
| Accumbens | −612.51 | −0.38 (112) | 0.707 | 1327.12 | 0.71 (111) | 0.477 | 59.5 | 0.03 (112) | 0.973 | −2164.56 | −1.26 (112) | 0.21 |
| Thalamus | 123.87 | 0.83 (112) | 0.409 | −105.72 | 0.63 (111) | 0.528 | 204.68 | 1.25 (112) | 0.215 | 18.97 | 0.11 (112) | 0.91 |
| Hippocampus | −286.2 | −1.02 (112) | 0.310 | −854.4 | −2.82 (111) | 0.006 ** | −387.8 | −1.27 (112) | 0.205 | −867.32 | −2.87 (112) | 0.005 ** |
| Amygdala | 184.8 | 0.18 (112) | 0.858 | −441.54 | −0.39 (111) | 0.698 | −1073.94 | −0.96 (112) | 0.337 | −1591.96 | −1.47 (112) | 0.144 |
| Corpus Callosum | 805.59 | 1.64 (112) | 0.105 | 1143.77 | 2.04 (111) | 0.044 * | −72.81 | −0.13 (112) | 0.898 | −190.95 | −0.32 (112) | 0.751 |
Note: beta† indicates estimated values for age and sex-adjusted interaction effect of Wechsler Adult Intelligence Scale 4th Edition (WAIS-IV) cognitive performance predicted by region of interest (ROI) volume.
Abbreviations: VCI, Verbal Comprehension Index; PRI, Perceptual Reasoning Index; WMI, Working Memory Index; PSI, Processing Speed Index
P < 0.05
P < 0.01
Table 2.
CTG repeat effect on cognitive performance in DM1-affected individuals.
| Effect | VCI |
PRI |
WMI |
PSI |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta† | t (df) | P | beta† | t (df) | P | beta† | t (df) | P | beta† | t (df) | P | |
| CTG repeat ‡ | −0.437 | −1.933 (47) | 0.0592 | −0.770 | −2.614 (47) | 0.0120* | −0.604 | −2.018 (47) | 0.0493* | −0.795 | −2.548 (47) | 0.0142* |
Note: beta† indicates estimated values from age-adjusted residuals for CTG repeat length as a predictor of cognitive performance on Wechsler Adult Intelligence Scale 4th Edition (WAIS-IV) indices.
CTG repeat ‡ indicates square-root transformed CTG repeat lengths.
Abbreviations: VCI, Verbal Comprehension Index; PRI, Perceptual Reasoning Index; WMI, Working Memory Index; PSI, Processing Speed Index
P < 0.05
P < 0.01
Figure 4:
Associations between cognitive functions and CTG repeat expansion in DM1-affected adults. To account for the skewed distribution, we took the square root of CTG repeat length in DM1-affected adults. The x-axes on the plots show age- and sex adjusted CTG residuals. Panel a shows the association with Verbal Comprehension, Panel b shows the association with Perceptual Reasoning, Panel c shows the association with Working Memory, and Panel d shows the association with Processing Speed.
Discussion
We report significant differences in cognitive performance between patients with DM1 and unaffected controls on measures of Working Memory, Processing Speed and Perceptual Reasoning. Variation in performance in the latter two domains was significantly associated with hippocampal volume, that is, enlarged hippocampal volume was associated with poorer performance on Processing Speed and Perceptual Reasoning in DM, but not in unaffected controls. These results are interesting in the context of Gallais et al.’s 2016 findings that muscular impairment at baseline did not predict changes in neuropsychological performance over time; the authors also noted that using MIRS as a global measurement of muscular impairment may not be sensitive enough to slight muscular changes to correlate with cognition.
In accordance with previous findings (Meola & Sansone, 2007), when compared with normative data, DM1 patients scored within the average range of cognitive performance. When compared to the unaffected adult control sample, however, DM1 patients scored significantly lower on measures of Working Memory, Processing Speed, and Perceptual Reasoning. In line with prior research (Baldanzi et al., 2016; Peric et al., 2017), we found no significant differences in Verbal Comprehension scores between adult DM1 patients and unaffected controls.
Previous studies have indicated visuospatial dysfunction in over 75% of DM1 patients, although the authors did not collect neuroimaging data for possible morphological correlations (Peric et al., 2017), however, some of these results may be due to muscle impairment. Our multi-site structural neuroimaging study showed evidence of increased hippocampus volume in individuals with DM1 relative to controls (van der Plas et al., 2019). Results from the present study suggest that difficulties in visuospatial domain may be associated with hippocampal enlargement in DM1.
Our findings indicated that individual aspects of cognitive performance were different between DM1-affected and unaffected individuals, including correlations with brain structure. In the context of brain volume, previous research in different populations have shown that bigger does not always mean better. For example, in autism spectrum disorder, larger brains may be associated with more severe cognitive impairments (Amaral et al., 2017). It is possible that these structural abnormalities are developmental in nature (van der Plas et al. 2019).
Our results are different from a previous study that used voxel-based morphometry to show that hippocampal degeneration is associated with decreased episodic memory in patients with DM1 (Weber et al., 2010). We used volumetric structural imaging in a sample over twice the size than the Weber et al. study, which could possibly account for the observed differences. Additionally, we did not find a significant correlation between hippocampal volume and memory function; however, our neuropsychological battery measured working memory as opposed to episodic memory that has classically been associated with hippocampal function (Marchetti, 2014; Quinette et al., 2006). These contrasting results may be the result of different parcellation methods, neuropsychological measures, and sample populations. Of note, previous studies have indicated a differentiation in structures relating to distinct types of memory (Marchetti, 2014; Quinette et al., 2006). Neuroimaging findings have indicated that the hippocampus may consolidate long-term memories (i.e. episodic memories), while the prefrontal cortex may be related to maintaining information over a short period of time (i.e. working memory; Marchetti, 2014; Quinette et al., 2006). These functional differences in structure may help explain why our findings of increased hippocampal volume in DM1-affected participants was not related to decreased Working Memory performance.
This study was not without limitations. Two different MRI scanners were used for imaging acquisition, although the differences were accounted for statistically. Our sample was slightly restricted due to the nature of DM1 as a degenerative muscular disease and did not contain any participants with a MIRS score of 5 (severe muscle impairment), therefore, this sample is likely not generalizable to the entire DM1 population. Additionally, we recognize the potential confounding factor of muscle manipulation required for some WAIS-IV subtests (i.e. Block Design, Symbol Search, and Coding). Because the indices of the WAIS-IV are not independent, a statistical limitation was multiple comparisons. Previous authors have utilized alternate measures of cognitive function to exclude motor biases that may occur in WAIS-IV (Censori et al., 1990). Finally, correlations between structure and function may hint at potential neurobiological substrates involved in cognitive processes, but future studies incorporating measures of brain activity via fMRI studies may be warranted.
In this study of adult-onset DM1, affected individuals scored significantly lower than unaffected age- and sex-matched controls on measures of Working Memory, Processing Speed, and Perceptual Reasoning. Processing Speed and Perceptual Reasoning performance were significantly predicted by the interaction between group and hippocampal volume, with potentially pathological enlargement correlating with poorer performance. Further research should be conducted to examine potential longitudinal changes in cognition and brain structure in DM1-affected patients.
Supplementary Material
Supplementary table 1: Kolomogorov-Smirnov test across scanners and groups. Note: KS Test was performed on Siemens vs. GE scanners.
Significance Statement.
Myotonic dystrophy (DM) is the muscular disease with the highest prevalence rate in humans. Myotonic dystrophy type 1 (DM1) is primarily thought of as a disease affecting muscular function; however, many patients also experience cognitive difficulties. We found that abnormalities in brain structure in DM1, particularly the hippocampus, contributed to visuospatial ability differences compared to unaffected controls. Results from this study may help find ways to improve cognitive problems in patients with DM1.
Acknowledgments
Grants information: NINDS #5R01NS094387-04; the Wyck Foundation
Footnotes
Conflict of Interest Statement
No authors have any conflicts of interest to declare.
Disclosure of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
Data Accessibility
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Amaral DG, Li D, Libero L, Solomon M, Van de Water J, Mastergeorge A, … Wu Nordahl C (2017). In pursuit of neurophenotypes: The consequences of having autism and a big brain. Autism Research, 10(5), 711–722. 10.1002/aur.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldanzi S, Cecchi P, Fabbri S, Pesaresi I, Simoncini C, Angelini C, … Siciliano G (2016). Relationship between neuropsychological impairment and grey and white matter changes in adult-onset myotonic dystrophy type 1. NeuroImage: Clinical, 12, 190–197. 10.1016/j.nicl.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callus E, Bertoldo EG, Beretta M, Boveri S, Cardani R, Fossati B, … Meola G (2018). Neuropsychological and psychological functioning aspects in myotonic dystrophy type 1 patients in Italy. Frontiers in Neurology, 9(SEP), 1–17. 10.3389/fneur.2018.00751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso F, Agosta F, Peric S, Rakočević-Stojanović V, Copetti M, Kostic VS, & Filippi M (2014). Cognitive impairment in myotonic dystrophy type 1 is associated with white matter damage. PLoS ONE, 9(8), 1–8. 10.1371/journal.pone.0104697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censori B, Danni M, Del Pesce M, & Provinciali L (1990). Neuropsychological profile in myotonic dystrophy. J Neurol, 237(4), 251–256. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2391548 [DOI] [PubMed] [Google Scholar]
- D’Angelo MG, & Bresolin N (2006). Cognitive impairment in neuromuscular disorders. Muscle and Nerve, 34(1), 16–33. 10.1002/mus.20535 [DOI] [PubMed] [Google Scholar]
- De Antonio M, Dogan C, Hamroun D, Mati M, Zerrouki S, Eymard B, … Bassez G (2016). Unravelling the myotonic dystrophy type 1 clinical spectrum: A systematic registry-based study with implications for disease classification. Revue Neurologique, 172(10), 572–580. 10.1016/j.neurol.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Douniol M, Jacquette A, Cohen D, Bodeau N, Rachidi L, Angeard N, … Guilé JM (2012). Psychiatric and cognitive phenotype of childhood myotonic dystrophy type 1. Developmental Medicine and Child Neurology, 54(10), 905–911. 10.1111/j.1469-8749.2012.04379.x [DOI] [PubMed] [Google Scholar]
- Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, … Shinohara RT (2018). Harmonization of cortical thickness measurements across scanners and sites. NeuroImage, 167(June 2017), 104–120. 10.1016/j.neuroimage.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallais B, Gagnon C, Mathieu J, & Richer L (2017). Cognitive decline over time in adults with myotonic dystrophy type 1: A 9-year longitudinal study. Neuromuscular Disorders, 27(1), 61–72. 10.1016/j.nmd.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Gourdon G, & Meola G (2017). Myotonic dystrophies: State of the art of new therapeutic developments for the CNS. Frontiers in Cellular Neuroscience, 11(April), 1–14. 10.3389/fncel.2017.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MJ, McLean J, Cumming S, Ballantyne B, McGhie J, Jampana R, … Farrugia ME (2018). Outcome measures for central nervous system evaluation in myotonic dystrophy type 1 may be confounded by deficits in motor function or insight. Frontiers in Neurology, 9(OCT). 10.3389/fneur.2018.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, & Rabinovic A (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8(1), 118–127. 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- Liu D, Johnson HJ, Long JD, Magnotta VA, & Paulsen JS (2014). The power-proportion method for intracranial volume correction in volumetric imaging analysis. Frontiers in Neuroscience, 8(OCT), 1–8. 10.3389/fnins.2014.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti G (2014). Attention and working memory: Two basic mechanisms for constructing temporal experiences. Frontiers in Psychology, 5(AUG), 1–15. 10.3389/fpsyg.2014.00880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Boivin H, Meunier D, Gaudreault M, & Bégin P (2001). Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology, 56(3), 336–340. 10.1212/WNL.56.3.336 [DOI] [PubMed] [Google Scholar]
- Meola G, & Cardani R (2015). Myotonic dystrophies: An update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1852(4), 594–606. 10.1016/j.bbadis.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Meola G, & Sansone V (2007). Cerebral involvement in myotonic dystrophies. Muscle and Nerve, 36(3), 294–306. 10.1002/mus.20800 [DOI] [PubMed] [Google Scholar]
- Minnerop M, Gliem C, & Kornblum C (2018). Current progress in CNS imaging of myotonic dystrophy. Frontiers in Neurology, 9(AUG). 10.3389/fneur.2018.00646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modoni A, Silvestri G, Grazia Pomponi M, Mangiola F, Tonali PA, & Marra C (2004). Characterization of the Pattern of Cognitive Impairment in Myotonic Dystrophy Type 1. Archives of Neurology, 61(12). 10.1001/archneur.61.12.1943 [DOI] [PubMed] [Google Scholar]
- Peric S, Rakocevic Stojanovic V, Mandic Stojmenovic G, Ilic V, Kovacevic M, Parojcic A, … Meola G (2016). Clusters of cognitive impairment among different phenotypes of myotonic dystrophy type 1 and type 2. Neurological Sciences, 38(3), 415–423. 10.1007/s10072-016-2778-4 [DOI] [PubMed] [Google Scholar]
- Pierson R, Johnson H, Harris G, Keefe H, Paulsen JS, Andreasen NC, & Magnotta VA (2011). Fully automated analysis using BRAINS: AutoWorkup. NeuroImage, 54(1), 328–336. 10.1016/j.neuroimage.2010.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinette P, Guillery-Girard B, Noël A, de la Sayette V, Viader F, Desgranges B, & Eustache F (2006). The relationship between working memory and episodic memory disorders in transient global amnesia. Neuropsychologia, 44(12), 2508–2519. 10.1016/j.neuropsychologia.2006.03.031 [DOI] [PubMed] [Google Scholar]
- Rubinsztein S, Rubinsztein DC, Mckenna PJ, Goodburn S, & Holland AJ (1997). Mild myotonic dystrophy is associated with memory impairment in the context of normal general intelligence. Journal of Medicine Genetics, 34, 229–233. 10.1136/jmg.34.3.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, & Gee JC (2010). N4ITK: Improved N3 bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. 10.1109/TMI.2010.2046908.N4ITK [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Plas E, Hamilton MJ, Miller JN, Koscik TR, Long JD, Cumming S, … Nopoulos PC (2019). Brain Structural Features of Myotonic Dystrophy Type 1 and their Relationship with CTG Repeats. Journal of Neuromuscular Diseases, 6(3), 321–332. 10.3233/JND-190397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorst M, Okkersen K, Kessels RPC, Meijer FJA, Monckton DG, van Engelen BGM, … Raaphorst J (2019). Structural white matter networks in myotonic dystrophy type 1. NeuroImage: Clinical, 21, 101615 10.1016/j.nicl.2018.101615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Suh JW, Das SR, Pluta JB, Craige C, & Yushkevich PA (2013). Multi-atlas segmentation with joint label fusion. IEEE Transactions on Pattern Analysis and Machine Intelligence, 35(3), 611–623. 10.1109/TPAMI.2012.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber YG, Wolf M, Roebling R, Hoffmann S, Walter H, Mottaghy FM, … Kassubek J (2010). Comparative analysis of brain structure, metabolism, and cognition in myotonic dystrophy 1 and 2. Neurology, 74(14), 1108–1117. 10.1212/wnl.0b013e3181d8c35f [DOI] [PubMed] [Google Scholar]
- Whitaker S (2013). Appendix I: The WISC-IV and WAIS-IV Subtests. Intellectual Disability: An Inability to Cope with an Intellectually Demanding World, 170–191. Retrieved from https://link.springer.com/content/pdf/bbm%3A978-1-137-02558-6%2F1.pdf [Google Scholar]
- Young Kim E, & Johnson HJ (2013). Robust multi-site MR data processing: iterative optimization of bias correction, tissue classification, and registration. Frontiers in Neuroinformatics, 7(November), 1–11. 10.3389/fninf.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1: Kolomogorov-Smirnov test across scanners and groups. Note: KS Test was performed on Siemens vs. GE scanners.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.