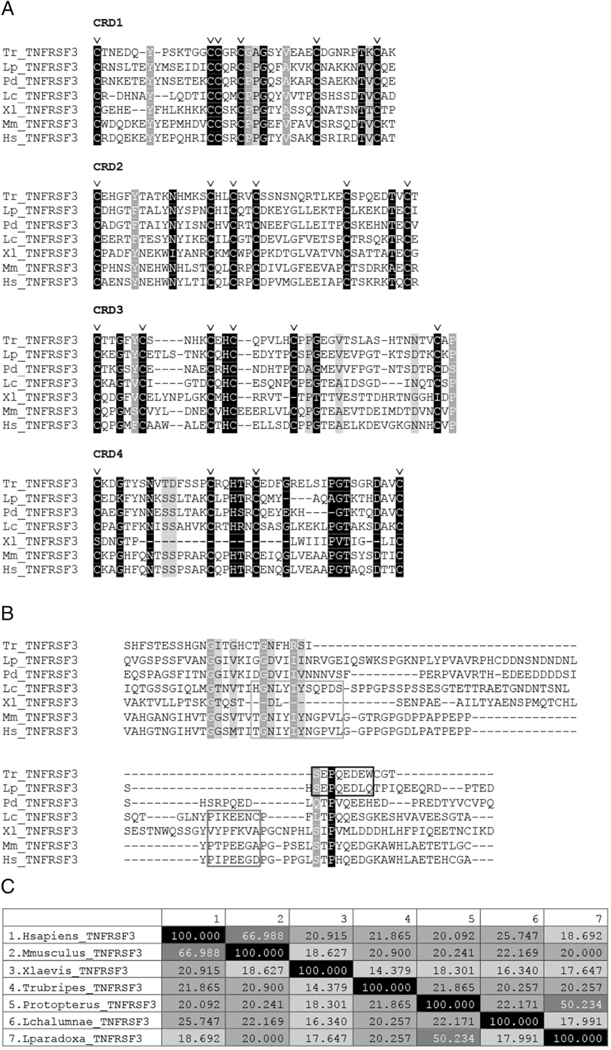
FIGURE 5.
Comparison of TNFRSF3 amino acid sequences among jawed vertebrates. (A) Extracellular domain of TNFRSF3, with triangles indicating extracellular CRD and residues required for ligand binding in mammals. (B) Alignment of TNFRSF3 intracellular domain. First box indicates unconventional TRAF binding motif, second box indicates conventional TRAF binding motif, and third box indicates a reported bony fish-specific conserved TRAF binding motif. (C) Amino acid sequence identity of TNFRSF3 molecules aligned in (A) and (B), darker shades of gray indicate higher percentage identity. The amino acids shaded in black, dark gray, and light gray denote conserved amino acids going from most conserved to least conserved across vertebrate lineage, respectively. The “v” above certain columns indicates the conserved cysteines that denote the TNFRSF motif.