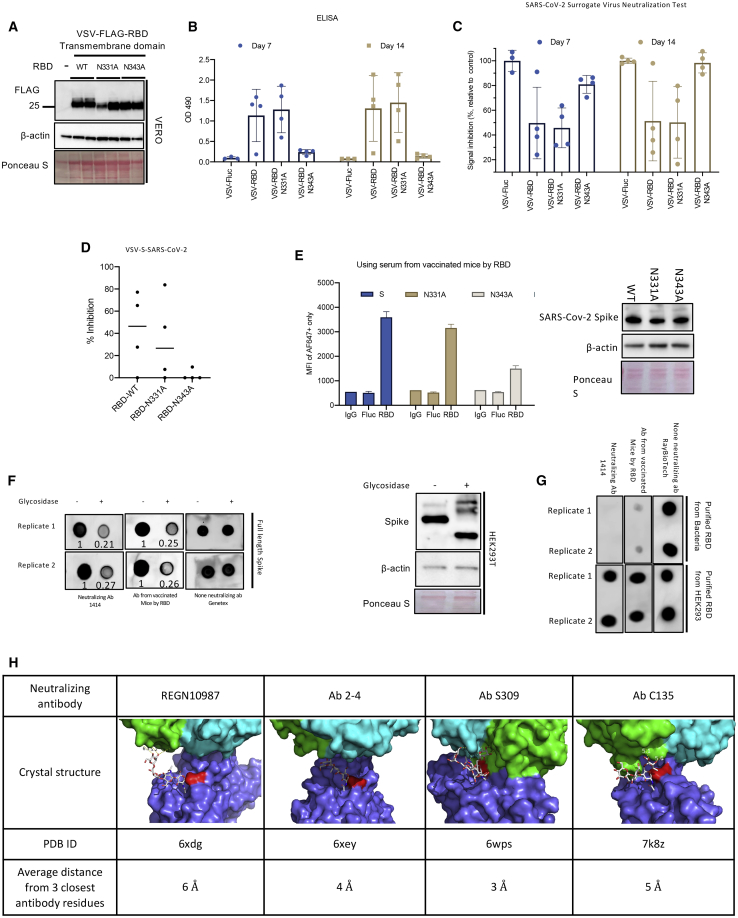
Figure 7.
N-linked glycosylation of RBD influences antigenicity and immunogenicity
(A) Immunoblot analysis of VSVΔ51-RBD-TMD-infected cell lysates. (B) Serum anti-RBD IgG levels of mice vaccinated with VSVΔ51-RBD-TMD WT and mutants were measured using ELISA. (C–E) Neutralizing antibody response was also measured using an ELISA-based surrogate neutralization assay (C) or a VSV-S pseudovirus-based assay (D) using 64-fold diluted serum. (E). HEK293T cells were transfected with expression constructs for WT, N331A, or N343A S and analyzed by flow cytometric analysis for S cell surface expression using sera from VSVΔ51-FLuc or VSVΔ51-RBD-TMD-WT vaccinated mice as a primary antibody. Immunoblot analysis showing total S expression in transfected cells is shown on the right panel. (F) Dot blot analyses of Endo F1/F2/F3-treated cell lysates probing with different RBD-targeted antibodies or sera from VSVΔ51-RBD-TMD-WT vaccinated mice. Immunoblot analyses showed the effect of glycosidase treatment on S protein migration (right panel). (G) Dot blot analyses comparing recognition of recombinant RBD purified from bacteria or HEK293 to different RBD-targeted antibodies or sera from VSVΔ51-RBD-TMD-WT-vaccinated mice. (H) Crystal structure of S complexed with four known neutralizing antibodies reveals the proximity of the binding interface near the RBD glycosites.