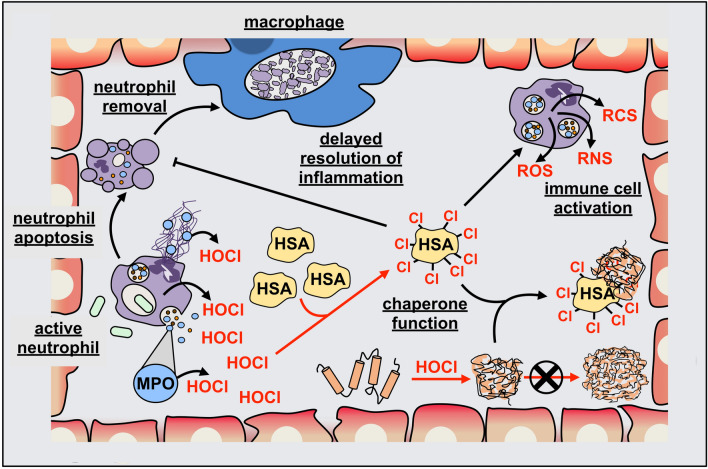
Fig. 4.
Immunomodulatory role of HOCl-modified human serum albumin (HSA) during infection and inflammation. At the site of infection or inflammation, activated neutrophils release myeloperoxidase (MPO; blue circle) into the extracellular surroundings via leakage during phagocytosis, degranulation or association with neutrophil extracellular traps (NETs). Extracellular MPO generates hypohalous acids, particularly hypochlorous acid (HOCl), in the immediate vicinity to host cells and host proteins, causing oxidative damage. Due to its high abundance in blood and interstitial fluid, HSA (yellow) is considered a major scavenger of HOCl and as such, becomes modified upon HOCl-stress. Reversible chlorination of its basic amino acid side-chains (N-chlorination) by HOCl results in the activation of HSA’s chaperone function, allowing HSA to effectively bind and protect other proteins from HOCl-induced aggregation. Moreover, N-chlorination also turns HSA into a potent activator of immune cells and thereby increases the generation of reactive oxygen, nitrogen or chlorine species (ROS/RNS/RCS) by these cells. Finally, HOCl-modified HSA can extend neutrophil lifespan at sites of infection and inflammation by inhibiting cell apoptosis and thus delays the removal of neutrophils by macrophages and the resolution of inflammation. Both, the persistent activation of neutrophils and neutrophil lifespan extension provide a potentially detrimental positive feedback loop that may ultimately lead to chronic inflammation