Figure 1.
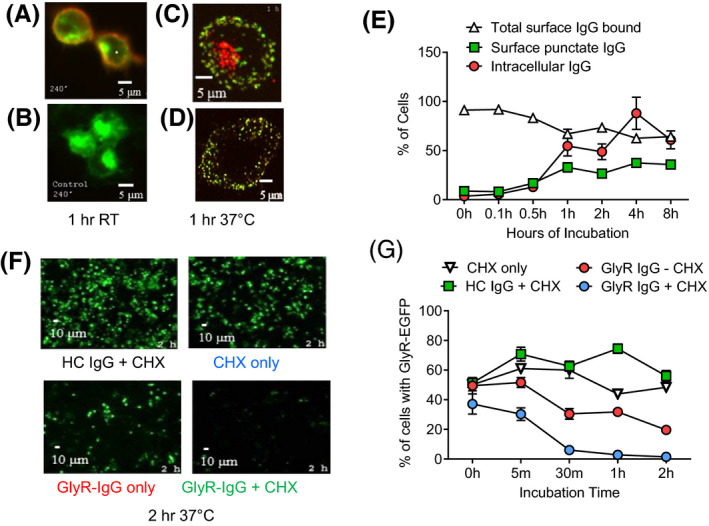
GlyR‐EGFP internalization caused by GlyR‐Ab is partially compensated by ongoing GlyR expression (a) GlyR‐Abs (red) bind to GlyR‐EGFP (green) HEK cells at 1 h room temperature compared with control IgG (b). At 37°C, GlyR‐Abs are internalized as detected after permeabilization (c) but are still present on the surface where IgG binds in a punctate pattern (d). Mean results from two sera showing increase of internal IgG and surface punctate IgG (Two‐sided Student’s t‐test t = 6308 df =8, P = 0.0002) over time with a slight decrease in total IgG bound (e) (Two‐sided Student’s t‐test t = 6308 df = 8, P = 0.0002). Incubation of GlyR‐EGFP (green) HEK cells with GlyR‐IgG over 2 h leads to a loss of EGFP‐GlyR compared to incubation with HC‐IgG (f). Results from two experiments from one sera shows a decrease of the proportion of GlyR‐EGFP cells when incubated with GlyR‐IgG at 37°C compared to incubation with HC‐IgG; the effect is even greater with cycloheximide treatment that prevents new GlyR synthesis (f, g) (Two‐way repeated measures ANOVA F (12, 80) = 8,883 P < 0.0001).
