Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has resulted in drastic changes to the practice of medicine, requiring healthcare systems to find solutions to reduce the risk of infection. Using a case series, we propose a protocol for same-day discharge (SDD) for selected patients undergoing transcatheter aortic valve replacement (TAVR) using real-time remote cardiac monitoring. Six patients with severe symptomatic aortic stenosis underwent TAVR and were discharged on the same day.
Case summary
Six patients with symptomatic severe native or bioprosthetic aortic valve stenosis underwent a successful transfemoral TAVR using standard procedures, including the use of rapid atrial pacing to assess the need for permanent pacemaker implantation. Following TAVR, patients were monitored on telemetry in the recovery area for 3 h, ambulated to assess vascular access stability, and discharged with real-time remote cardiac monitoring if no new conduction abnormality was observed. The patients were seen by tele-visits within 2 days and 2 weeks after discharge.
Discussion
Amidst the COVID-19 pandemic, SDD following successful transfemoral TAVR may be feasible for selected patients and reduce potential COVID-19 exposure.
Keywords: Transcatheter aortic valve replacement, Same-day discharge, Rapid atrial pacing, Case series, COVID-19
Learning points
Same-day discharges after a transcatheter aortic valve replacement (TAVR) is feasible in the presence of real-time remote cardiac monitoring.
Rapid atrial pacing can be used to select patients for same-day discharge protocol post-TAVR.
Real-time remote cardiac monitoring is an integral tool for a safe same-day discharge TAVR protocol.
Same-day discharge to selected patients might be of benefit in preventing potential exposure during the COVID-19 pandemic.
Introduction
Amidst the coronavirus disease 2019 (COVID-19) pandemic, governments across the world have taken measures to contain the spread of the disease resulting in unique pressures on healthcare systems to find solutions to deliver care and mitigate the risk of infection.1 Initially, the Center for Disease Control recommended postponing elective procedures to prevent unnecessary exposure and infection to the patients and healthcare workers.2 The current pandemic coupled with a need to minimize the risk for COVID-19 infection has led many physicians and hospitals to revise current procedural protocols to reduce hospital admission, length of stay, and limit exposure while still continuing to provide evidence-based treatment options.3,4
Transcatheter aortic valve replacement (TAVR) is a well-established alternative to surgical aortic valve replacement for the treatment of severe aortic stenosis (AS) or bioprosthetic aortic valve dysfunction.5 Despite reductions in a majority of periprocedural complications (e.g. death, stroke, bleeding, and major vascular), complete heart block (CHB) and/or high-degree atrioventricular block (HAVB) requiring permanent pacemaker placement remain a persistent limitation of TAVR.6 With a growing trend towards using routine conscious sedation, early ambulation and recovery is feasible following TAVR and rhythm monitoring (24–48 h) is typically the major barrier for early discharge. A single case report of a patient being discharged 6 h after TAVR has been reported previously.7 In light of the current pandemic, we implemented a same-day discharge (SDD) protocol for select patients following TAVR.
Timeline
| Outpatient | All six patients were seen in the outpatient clinic with complaints of shortness of breath; workup revealed severe symptomatic aortic valve stenosis. Patients qualified for transcatheter aortic valve replacement (TAVR) |
| Operative procedure | Arrived at the pre-/post-procedure recovery area and pre-operative ECG was performed. Patients undergo standard TAVR procedures and are monitored in the recovery area. Two patients were identified for rapid atrial pacing (RAP) post-procedure; no Wenckebach on RAP and ECG unchanged from baseline without pre-existing left bundle branch block. All six patients were discharged on the same day after 3 h (ambulated multiple times to assess vascular stability) with real-time remote cardiac monitoring. |
| Post-procedure | Tele-visit the next day, 2 weeks, and 30 days later following TAVR with no complication and significant symptomatic resolution. |
Case series
We present a case series of six patients who underwent transfemoral TAVR and were discharged the same day with a real-time remote heart rhythm monitor (BodyGuardian® Mini—a single-lead monitor patch), which allows for continuous rhythm monitoring with an alert system for the presence of CHB/HAVB.8 The description of the details of each case is presented in Table 1. The decision to undergo TAVR was made following a multidisciplinary Heart team discussion and patient preference. The SDD protocol was developed in response to the COVID-19 pandemic to reduce healthcare exposure for necessary hospital procedures. All patients tested negative for COVID-19 prior to the procedure. All patients considered for SDD included those who were ambulatory and living independently with adequate social support at home and/or from supervised facilities
Table 1.
Details of TAVR cases
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Age | 94 | 57 | 86 | 72 | 80 | 74 |
| Sex | Female | Male | Male | Male | Male | Male |
| Hypertension | Yes | No | Yes | Yes | Yes | Yes |
| Coronary artery disease | Yes | No | Yes | Yes | Yes | Yes |
| Diabetes mellitus | No | No | No | No | No | Yes |
| CKD | No | No | No | Yes | No | No |
| Symptoms | Chest pain at rest, exertional dyspnoea | Exertional dyspnoea | Exertional dyspnoea | Exertional fatigue and chest pain | Lightheadedness, Dizziness | Exertional fatigue and chest pain |
| Exam | Crescendo-decrescendo systolic murmur | Crescendo-decrescendo systolic murmur | Crescendo-decrescendo systolic murmur | Crescendo-decrescendo systolic murmur | Crescendo-decrescendo systolic murmur and decrescendo diastolic murmur | Crescendo-decrescendo systolic murmur |
| NYHA/AHA | III/C | III/C | II/C | III/C | II/C | III/C |
| Ejection fraction | 60% | 55% | 60% | 55% | 60% | 75% |
| Indications | Symptomatic severe bioprosthetic valve dysfunction | Symptomatic severe low-flow/low-gradient AS in a native Bicuspid AV | Symptomatic severe high-gradient AS | Symptomatic severe high- gradient bicuspid AS | Symptomatic severe AS and moderate aortic insufficiency | Symptomatic severe high- gradient AS |
| STS/Euroscore II Score (%) | 5.6/16.1 | 1.2/0.9 | 4.6/2.2 | 1.3/1.8 | 1.6/1.2 | 1.7/2.3 |
| Aortic valve area | 0.25 cm2 | 0.9 cm2 | 0.23 cm2 | 0.75 cm2 | 0.9 cm2 | 1.0 cm2 |
| Aortic valve mean gradient | 22 mmHg | 30 mmHg | 45 mmHg | 39 mmHg | 32 mmHg | 51 mmHg |
| Aortic valve peak gradient | 40 mmHg | 56 mmHg | 77 mmHg | 69 mmHg | 48 mmHg | 81 mmHg |
| Aortic valve peak velocity | 3.2 m/s | 3.75 m/s | 4.3 m/s | 4.2 m/s | 2.9 m/s | 4.5 m/s |
| Procedure performed | Valve-in-valve TAVR using a 23 mm Sapien Ultra valve with bioprosthetic valve fracturing | TAVR using a 29 mm Sapien 3 valve | TAVR using a 23 mm Sapien Ultra | TAVR using a 29 mm Sapien 3 valve | TAVR using a 29 mm Sapien 3 valve | TAVR using a 26 mm Sapien Ultra valve |
| Pre-procedure ECG rhythm/PR/QRS | LBBB with first degree AV block/220/156 | NSR/148/96 | NSR/178/84 | NSR/180/100 | NSR with first-degree AV block/210/114 | NSR/170/90 |
| RAP performed | No | No | No | No | Yes | Yes |
| Transient heart block after TAVR | No | No | New-onset LBBB | No | No | No |
| Wenckebach with RAP | — | — | — | — | 110 beats per minute | No |
| Post-procedure ECG rhythm/PR/QRS | LBBB with first degree AV block/236/158 | NSR/154/96 | New-onset LBBB/202/154 | NSR/200/100 | NSR with first-degree AV block/204/114 | NSR/186/108 |
| Post-procedure complications | None | None | New-onset LBBB, underwent EP study—Normal HV conduction | None | None | None |
| Discharge | SDD with Body guardian | SDD with Body guardian | SDD with Body guardian | SDD with Body guardian | SDD with Body guardian | SDD with Body guardian |
| Post-discharge follow-up | No events recorded on BodyGuardian in 14 days | No events recorded on BodyGuardian in 14 days | No events recorded on BodyGuardian in 14 days | No events recorded on BodyGuardian in 14 days | No events recorded on BodyGuardian in 14 days | No events recorded on BodyGuardian in 14 days |
AHA, American Heart Association Heart Failure Classification; AS, aortic stenosis; ECG, electrocardiogram; EP, electrophysiology; LBBB, left bundle branch block; NSR, normal sinus rhythm; NYHA, New York Heart Association Heart Failure Classification; RAP, rapid atrial pacing; SDD, same-day discharge; STS, Society of Thoracic Surgery; TAVR, transcatheter aortic valve intervention.
A 94-year-old female with severe symptomatic bioprosthetic valve dysfunction underwent valve-in-valve TAVR with no post-procedural complications.
A 57-year-old male with severe symptomatic bicuspid low-flow low-gradient severe AS underwent TAVR with no post-procedural complications.
An 87-year-old female with severe symptomatic high-gradient severe AS underwent TAVR. Immediately post-procedure, she developed a new left bundle branch block and underwent an electrophysiology study suggesting normal AV node and bundle of His conduction.
A 72-year-old male with severe symptomatic high-grade bicuspid aortic stenosis underwent TAVR with no post-procedural complications.
An 80-year-old male with symptomatic mixed severe aortic stenosis and moderate aortic insufficiency underwent TAVR with no post-procedural complications. Rapid atrial pacing as performed post-TAVR, and Wenckebach developed at a paced rate of 110 beats per minute.
A 74-year-old male with severe symptomatic high-gradient AS underwent TAVR with no post-procedural complications. Rapid atrial pacing was performed post-TAVR without evidence of Wenckebach.
All six patients were discharged with a real-time remote heart rhythm monitor for 14 days post-procedure and recovered without complications. These patients were seen by tele-visits within 2 days and 2 weeks of discharge and reported significant symptomatic improvement. None of the patients had any complications.
Discussion
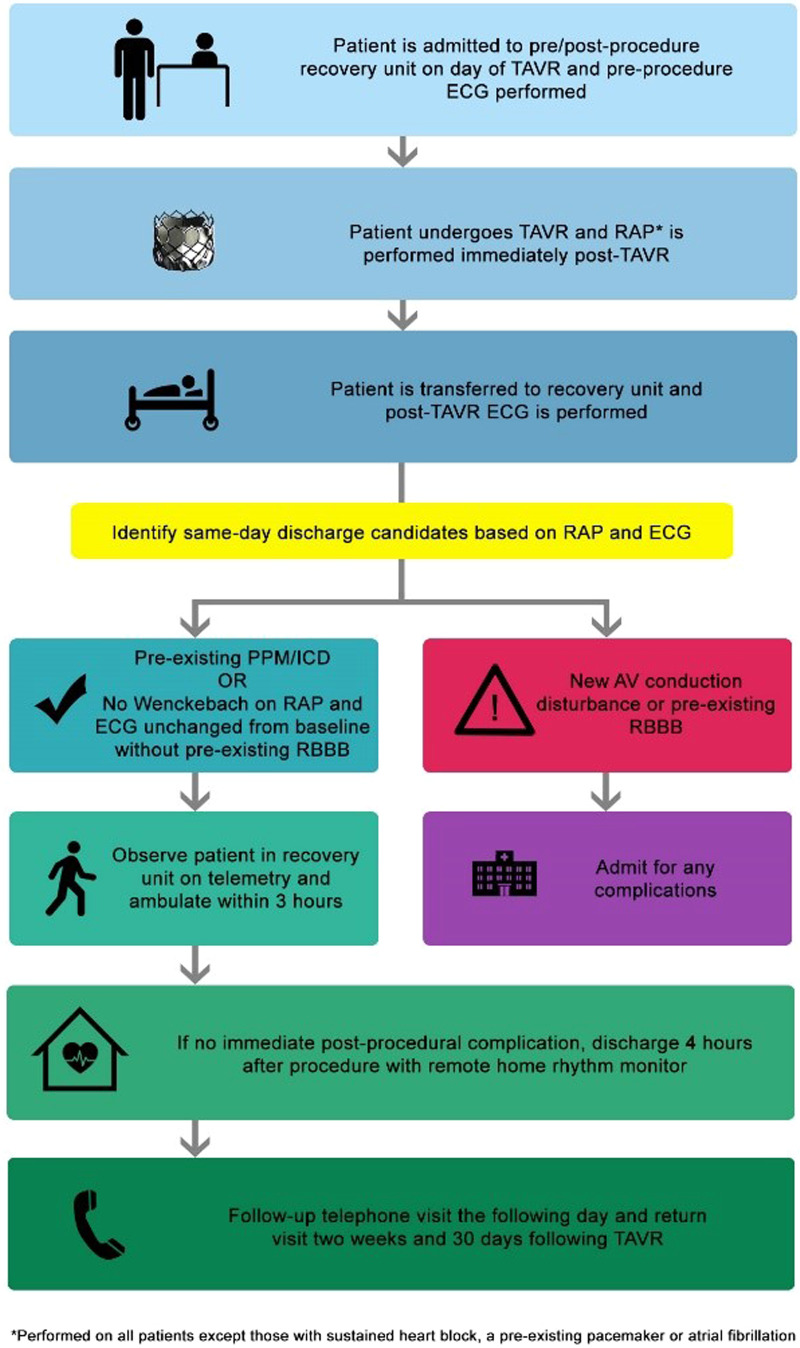
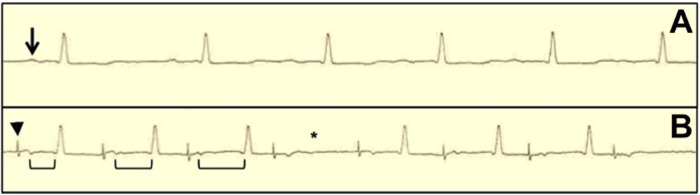
Following transfemoral TAVR, discharge within 1–2 days is achievable in most patients. The major impediment to early discharge following TAVR is the need for further rhythm monitoring to determine the need for pacemaker implantation. However, SDD is feasible in patients with a pre-existing permanent pacemaker/implantable cardioverter-defibrillator (PPM/ICD) or with the use of a real-time remote heart rhythm monitor, as demonstrated in our case series. Using a rhythm monitoring system that is continuously monitored with the capability of alerting both the patient and physicians immediately of any conduction disturbances is invaluable in establishing routine SDD for selected TAVR patients. Our proposed SDD protocol for TAVR is presented in Figure 1. The use of rapid atrial pacing (RAP) immediately following TAVR has a 99% negative predictive value for pacemaker implantation and can help risk-stratify patients in the management of post-TAVR conduction disturbances.9 We incorporated RAP (i.e. pacing wire is positioned in the right atrium and pacing is performed starting at 70 beats per minute and increased by 10 beats per minute every 20 beats to a maximum of 120 beats per minute to assess for the development of Wenckebach—Figure 2) into our TAVR procedural protocol shortly after publication and currently perform measurements on all patients who do not have sustained heart block, pre-existing pacemaker, or chronic atrial fibrillation.9 In our case series, RAP was performed on the last two of the six SDD cases and all patients were discharged with remote monitoring (Figure 3 shows the results of remote monitoring). Patient 5 developed Wenckebach at 110 beats per minute. The positive predictive value for pacemaker implantation is quite poor for patients who develop Wenckebach with RAP, where only 13% of patients who develop Wenckebach ultimately undergoing pacemaker implantation, thus, we discharged Patient 5 with remote monitoring.9 Patient 5 had a normal ECG pre-TAVR and was unchanged following the procedure. Given the absence of any underlying conduction disturbance on the pre-and-post-TAVR ECG, we felt this patient could be safely discharged on the same day. A negative RAP test (i.e. no Wenckebach) is very informative due to its strong negative predictive value. However, a positive RAP test is less helpful, and SDD candidacy should rely more on a careful assessment of the pre-and-post-TAVR ECGs.
Figure 1.
Transcatheter aortic valve replacement same-day discharge protocol.
Figure 2.
Rapid atrial pacing-induced Wenchebach atrioventricular block in patient immediately after transcatheter aortic valve replacement. (A) Baseline sinus rhythm at 60 beats/min (arrow denotes P-wave). (B) Wenckebach atrioventricular block (AVB) with rapid atrial pacing (RAP) at 90 beats/min (arrowhead denotes pacing spike, brackets denote prolonging PR interval, and asterisk denotes missed atrioventricular conduction) (image from Krishnaswamy et al.9).
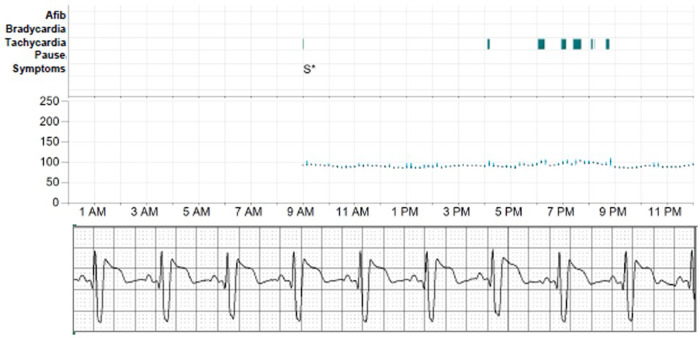
Figure 3.
Top panel shows the heart rate in the real-time along with the rhythm; the bottom panel shows the single-lead electrocardiogram as recorded via real time remote monitoring.
As shown in Figure 1, we identify patients without a pre-existing PPM/ICD for SDD based on a comparison of the pre-and-post-TAVR ECG (i.e. post-TAVR ECG unchanged from baseline) and the results of the RAP study. In our case series, Patient 3 was an exception to our proposed protocol. Patient 3 developed a new left bundle branch block (LBBB) with a QRS interval of 154 ms following TAVR. Traditionally, we would monitor patients with a new LBBB overnight and consider performing an electrophysiology (EP) study to assess the HV interval based on the ECG the day after the procedure. In this case, an EP study was performed on the same day as the TAVR procedure and was normal. Thus, we felt the patient could be safely discharged on the same day. Despite the utility of an EP study in post-TAVR conduction disturbances, routine performance of an EP study on the same day as TAVR has the potential for a false-positive result due to transient conduction disturbances that may occur from trauma and subsequent oedema related to the procedure, which can resolve over time. At this time, patients with a new LBBB may not be ideal candidates for SDD. The use of RAP may be helpful in patients with a new LBBB as the risk of pacemaker implantation was low in patients who did not develop Wenckebach with post-TAVR RAP.9 However, further investigation is needed to determine if using RAP can help further risk-stratify patients with a new LBBB and identify them as potential SDD candidates.
Early discharge (<3 days) following transfemoral TAVR is safe without any increase in post-procedural complications.10,11 Next-day discharge predictors after TAVR include male gender, younger age (79 ± 8.7 years), absence of atrial fibrillation, and lower serum creatinine.12 The majority of our patients also had these predictors of next-day discharge for TAVR, which suggests that these predictors may be applicable for SDD.12 Patients with a pre-existing PPM or ICD should be considered SDD candidates in the absence of any procedural complications, and it may be advantageous to schedule these patients accordingly (i.e. first or second case), given the high likelihood for SDD. Since an underlying right bundle branch block (RBBB) is the strongest predictor for PPM following TAVR, we feel strongly that patients with a pre-existing RBBB should not be discharged on the same day as their procedure. After identification, potential SDD candidates are recovered on telemetry in the catheterization laboratory pre-/post-procedure recovery unit, ambulated within 3 h of the procedure, and discharged 4 h after the procedure with a real-time remote home rhythm monitor. In our case series, all patients underwent TAVR using a balloon-expandable valve (i.e. SAPIEN 3 or Ultra). Thus, our protocol may only apply to balloon-expandable valve systems due to higher rates of PPM implantation in non-balloon-expandable valve systems (e.g. self-expanding system).13
The advantages of SDD include a shorter length of stay, which improves resource utilization, enhances patient satisfaction, and, in the current era, minimizes potential COVID-19 exposure. The disadvantages include the inability to provide an immediate direct assessment of patient with any post-procedural complication. Albeit, we feel the latter risk should be significantly minimized by selecting potential candidates for SDD. The COVID-19 pandemic has challenged our current practices of care, and solutions such as SDD TAVR can reduce the risk of infection, reduce hospital costs/resources, and increase patient satisfaction. Further prospective studies are needed to determine if these practices should be routinely used following the COVID-19 pandemic.
Lead author biography

Jeremiah P. Depta is the Director of the Advanced Valvular and Structural Heart Disease Program at Sands-Constellation Heart Institute at Rochester General Hospital in Rochester, New York. He serves as a proctor for TAVR instructing physicians across the U.S. and abroad. He completed his residency at Cleveland Clinic, General Cardiology Fellowship at Washington University in St Louis, and Interventional Cardiology/Structural Fellowship at Brigham and Women’s Hospital/Harvard Medical School. He serves as the principal site investigator for several multisenter randomized controlled trials with multiple publication in peer reviewed journals.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest J.P.D. discloses the following relationships—Consultant/Advisory Board: Edwards Lifesciences, Boston Scientific, WL Gore & Associates. D.L.B. discloses the following relationships—Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Level Ex, MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda.
Funding: none declared.
Supplementary Material
References
- 1. Wang X, Bhatt DL.. COVID-19: an unintended force for medical revolution? J Invasive Cardiol 2020;32:E81–E82. [PubMed] [Google Scholar]
- 2. American Hospital Association. Coronavirus update: New Information on Elective Surgery, PPE Conservation and Additional COVID-19 Issues. https://www.aha.org/advisory/2020-03-19-coronavirus-update-new-information-elective-surgery-ppe-conservation (8 July 2020).
- 3. Shah PB, Welt FGP, Mahmud E, Phillips A, Kleiman NS, Young MN, et al. ; American College of Cardiology and the Society for Cardiovascular Angiography and Interventions. Triage considerations for patients referred for structural heart disease intervention during the COVID-19 pandemic: an ACC/SCAI position statement. JACC Cardiovasc Interv 2020;13:1484–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan BE-X, Depta JP, Baibhav B, Bhatt DL.. Necessity of 45-day transesophageal echocardiography after the WATCHMAN procedure amid the COVID-19 pandemic. JACC: Cardiovasc Imaging 2020;13:2461–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA. et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 6. Rodés-Cabau J, Ellenbogen KA, Krahn AD, Latib A, Mack M, Mittal S. et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC Scientific Expert Panel. J Am Coll Cardiol 2019;74:1086–1106. [DOI] [PubMed] [Google Scholar]
- 7. Généreux P, Demers P, Poulin F.. Same day discharge after transcatheter aortic valve replacement: are we there yet? Cathet Cardiovasc Intervent 2016;87:980–982. [DOI] [PubMed] [Google Scholar]
- 8.Preventice Solutions Body Guardian HEART. https://www.preventicesolutions.com/patients/body-guardian-heart (8 July 2020).
- 9. Krishnaswamy A, Sammour Y, Mangieri A, Kadri A, Karrthik A, Banerjee K. et al. The utility of rapid atrial pacing immediately post-TAVR to predict the need for pacemaker implantation. JACC Cardiovasc Interv 2020;13:1046–1054. [DOI] [PubMed] [Google Scholar]
- 10. Kotronias RA, Teitelbaum M, Webb JG, Mylotte D, Barbanti M, Wood DA. et al. Early versus standard discharge after transcatheter aortic valve replacement: a systematic review and meta-analysis. JACC Cardiovasc Interv 2018;11:1759–1771. [DOI] [PubMed] [Google Scholar]
- 11. Barbanti M, van Mourik MS, Spence MS, Icovelli F, Martinelli GL, Muir DF. et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention 2019;15:147–154. [DOI] [PubMed] [Google Scholar]
- 12. Kamioka N, Wells J, Keegan P, Lerakis S, Binongo J, Corrigan F. et al. Predictors and clinical outcomes of next-day discharge after minimalist transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv 2018;11:107–115. [DOI] [PubMed] [Google Scholar]
- 13. Van Belle E, Vincent F, Labreuche J, Auffret V, Debry N, Lefèvre T. et al. Balloon-expandable versus self-expanding transcatheter aortic valve replacement: a propensity-matched comparison from the FRANCE-TAVI registry. Circulation 2020;141:243–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.