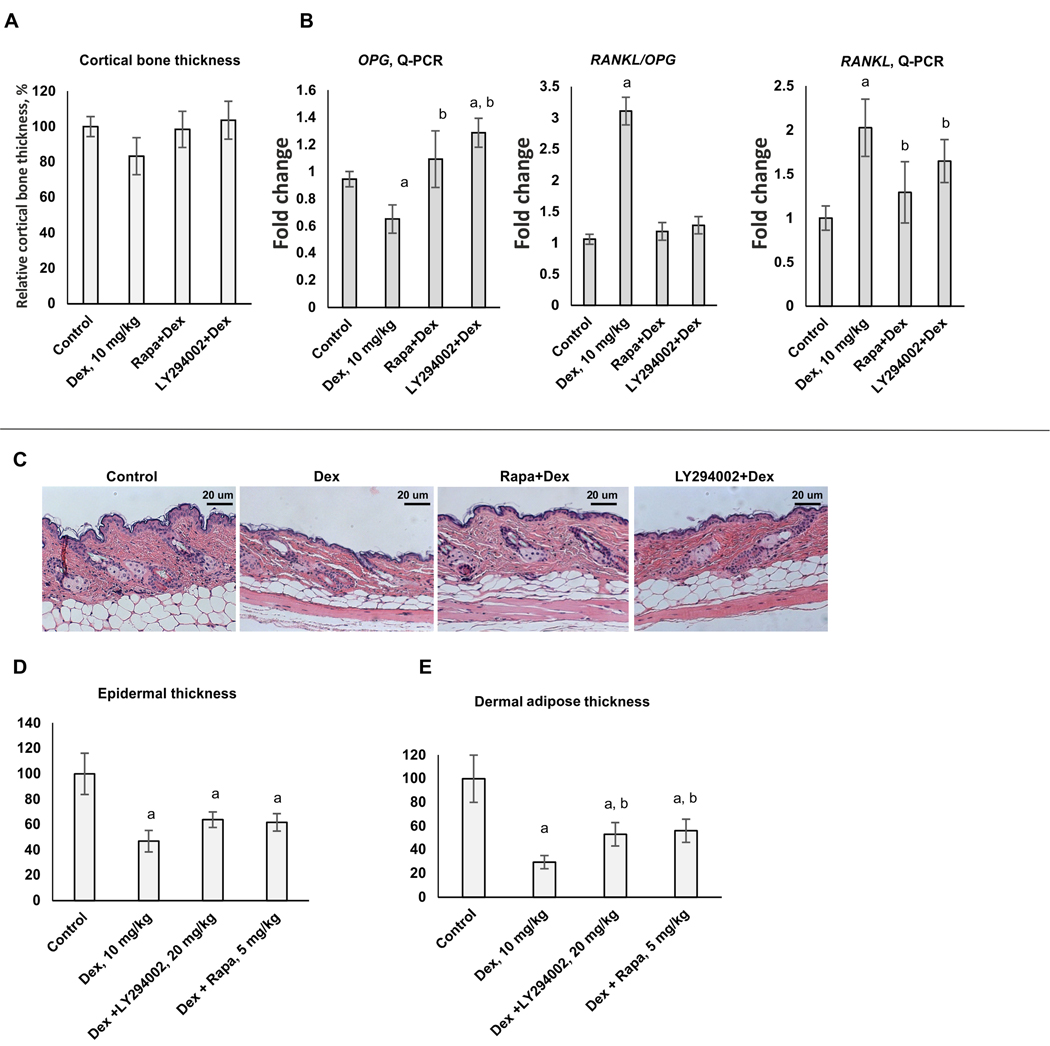
Figure 5. Protective effect of LY294002 and Rapamycin against atrophic effects of systemic chronic Dex treatment in bone and skin.
12 weeks old female BALB/c mice (10/group) were treated every 48 h with i.p. injections of LY294002 (20 mg/kg), Rapamycin (5 mg/kg) or vehicle (30% PEG3350, 4% DMSO, 5% Tween-20 in PBS) followed by i.p. injections of Dex (10 mg/kg) of vehicle (5% DMSO in PBS) 6 h later for 5 weeks. (A) Quantification of cortical bone thickness. (B) Q-PCR analysis of Rankl and Opg mRNA expression in bone tissue. The Q-PCR results (for three individual RNA samples/condition) were normalized to the expression of housekeeping gene RPL27, and presented as fold change compared to control. (C) H&E skin staining. (D, E) Quantification of the epidermal (D) and dermal adipose (E) thickness as described in Materials and Methods. In bar graphs the mean +/− SD is presented. Statistically significant differences as compared to: a-control; b-Dex (p<0.001) as and where indicated.